Long-acting anti-flu medical mask
An anti-influenza and mask technology, which is applied in the field of long-acting anti-influenza medical masks, can solve the problems of not killing viruses, and achieve high conservatism, inhibition of survival and transmission, and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The synthesis of embodiment 1 polypeptide
[0018] The 9-fluorenylmethoxycarbonyl (Fmoc) synthesis strategy was used to synthesize from the C-terminal to the N-terminal. 10mg of Rink-Amide-Resin resin (AAPPTec, product number RRZ001) was used as a carrier, and the active group of the carrier itself was connected to the carboxyl group of 5mg of the first amino acid (Fmoc-Q-NH2) protected by Fmoc.
[0019] Rinse the resin with N-methylpyrrolephrine (NMP) to remove redundant protected amino acids, add 20% piperidine / NMP solution (volume fraction) to the reactor (solid phase synthesizer) to remove the Fmoc group, react for 20min, Empty the reactor, wash the resin with 5mL NMP shaking, repeat 3 times, remove the Fmoc protection of the first amino acid residue; the exposed active amino group is connected to the carboxyl group of the next amino acid (5mg) that is amino-protected by Fmoc , forming the first peptide bond (Q-H). The above steps in this section are repeated (the...
Embodiment 2
[0020] Example 2 protein binding experiment
[0021] A / Brisbane / 10 / 2007(H3) HA protein, A / California / 04 / 2009(H1N1) HA protein, A / Canada / 720 / 2005(H2N2) HA protein, A / Anhui / 1 / 2005(H5N1) ) HA protein, A / Netherlands / 219 / 03 (H7N7) HA protein mixed with coating solution (synthetic peptide with a final concentration of 100ng / mL) coated in 96-well plate, overnight at 4°C, 1% BSA / PBS at 37 ℃ block 1h. Washed with PBST for 3 times, the serum after the first immunization was used as the primary antibody, diluted in PBST, the first dilution was 1:200, 5-fold serial dilution, and incubated at 37°C for 1.5h. Wash 3 times with PBST, the secondary antibody is HRP-labeled mouse secondary antibody, diluted in PBST, the dilution ratio is 1:3000, incubate at 37°C for 45min, wash 3 times with PBST, add TMB substrate for color development, at OD 450 UV light was detected at the place where human serum albumin was used as a control. The results are shown in Table 1 below.
[0022] Table 1 The bi...
Embodiment 3
[0024] The preparation of embodiment 3 masks
[0025]The preparation of nanofiber layer: add 1 part (parts by weight) polyamine and guanidinium salt polymer in the raw material of making gauze, 4 parts (parts by weight) polypropylene special material, 95 parts (parts by weight) polypropylene vinyl resin , mixed at 500°C for 45 minutes and granulated to form an antiviral matrix resin. The resin was processed into nanofibers with a diameter of 50nm and then processed into a sheet, and immersed in a concentration of 330ppm or 350ppm of nano-silver nitrate for 1h, followed by drying, then immersing in the antiviral polypeptide solution prepared in Example 1 for 30min at 45 degrees Celsius, and drying;
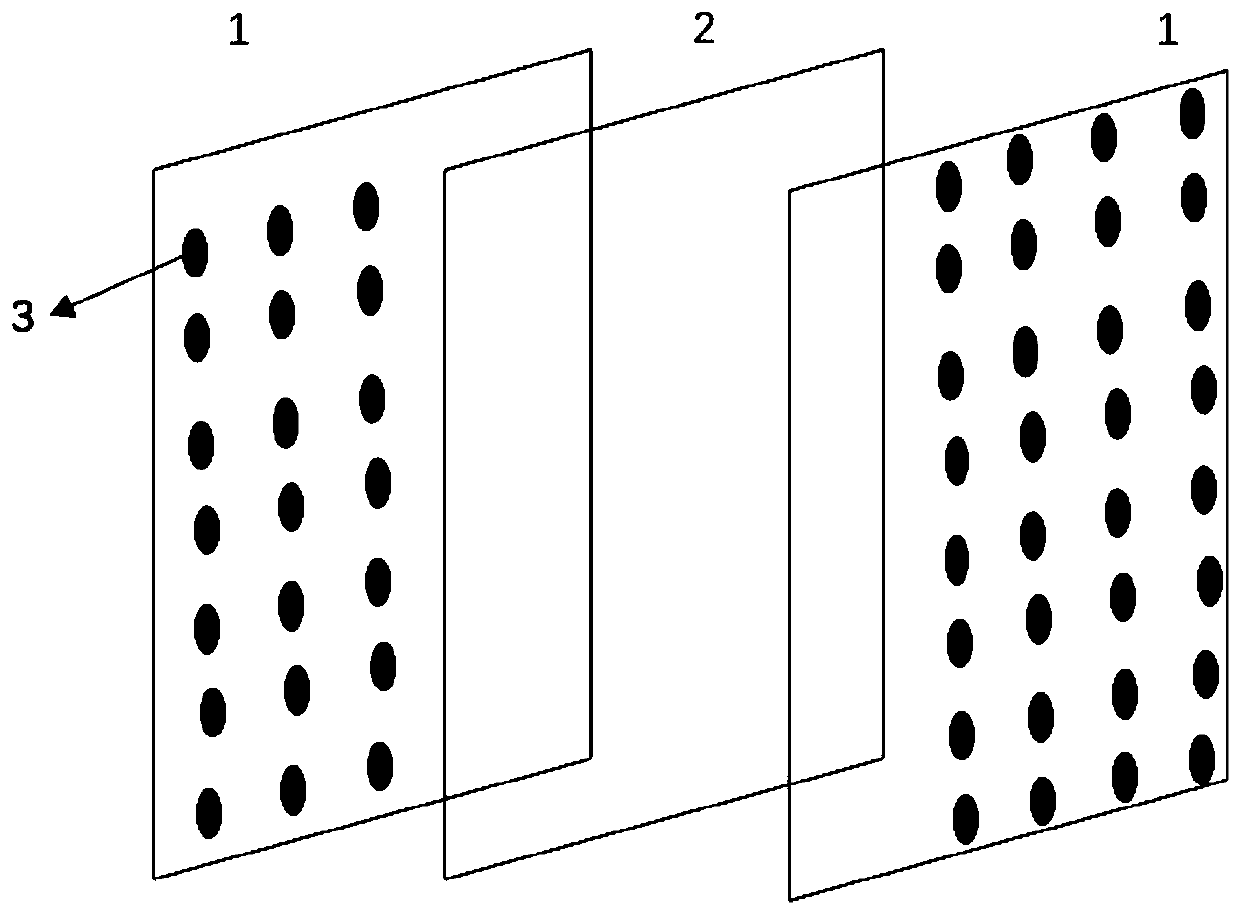
[0026] Preparation of the mask: Take the non-woven fabric sheet and laminate it on both sides of the nanofiber layer prepared above, and then press and form it at high temperature after 2 repeated superimpositions. The non-woven fabric sheet is provided with regularly formed throug...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com