Method for determining concentrations of vancomycin and degradation product in human serum
A vancomycin and degradation product technology, applied in the detection of drug concentration in human serum, vancomycin and crystalline degradation product CDP-1 concentration field, can solve the problem of inability to quantify degradation products separately, difficult to meet TDM requirements, difficult to separate standards It can achieve the effect of good recovery rate, low cost and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Instruments, materials and reagents:
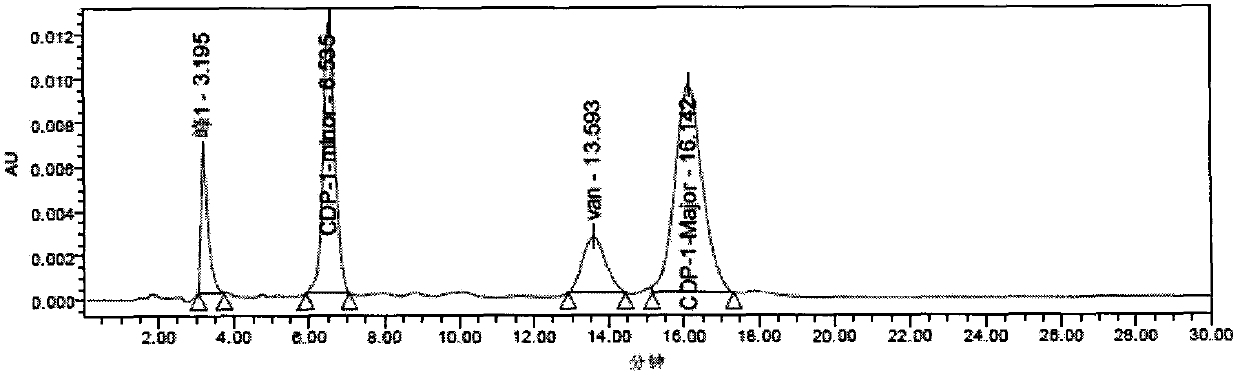
[0046] Instrument: Waters liquid chromatograph, equipped with Waters 2487 ultraviolet detector and Empower data acquisition and processing software, Waters Corporation of the United States;
[0047] Materials and reagents: CDP-1 standard product, batch number 1-TMH-108-1, purchased from Canada TRC Company; vancomycin, specification 1007u / mg, batch number 130360-201302, purchased from China Institute for the Control of Pharmaceutical and Biological Products; Potassium hydrogen and phosphoric acid, analytically pure, were purchased from Lingfeng Reagent Co., Ltd.; acetonitrile, chromatographically pure, purchased from Sigma-Aldrich; ultrapure water, freshly prepared daily by Milli-Q;
[0048] Experimental part:
[0049] Solution preparation: Accurately weigh an appropriate amount of CDP-1 standard substance into a 5mL volumetric flask, add ultrapure water to prepare a 1053μg / mL stock solution. Use 5% acetonitrile water to dilute to...
Embodiment 2
[0055] Instruments, materials and reagents:
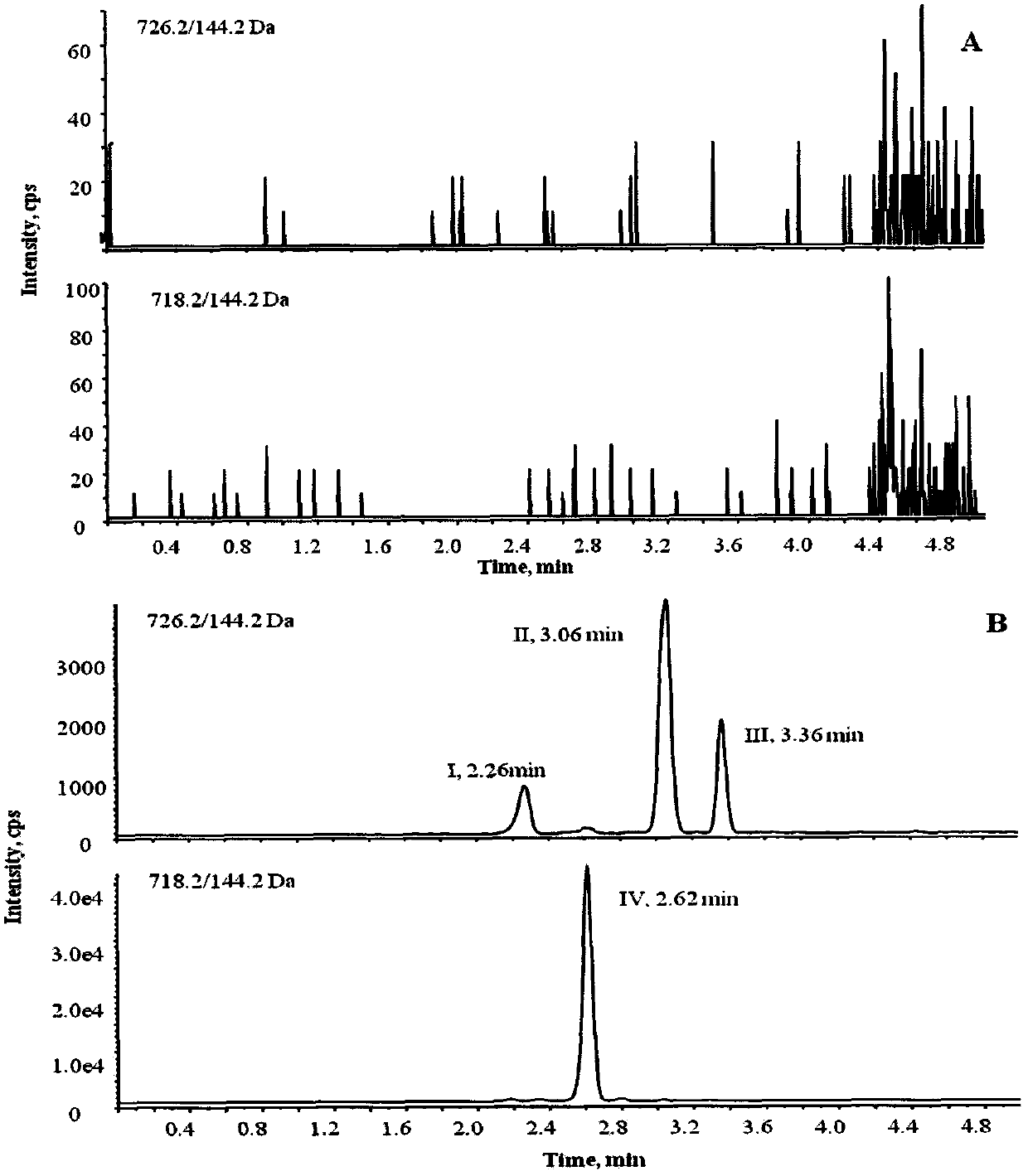
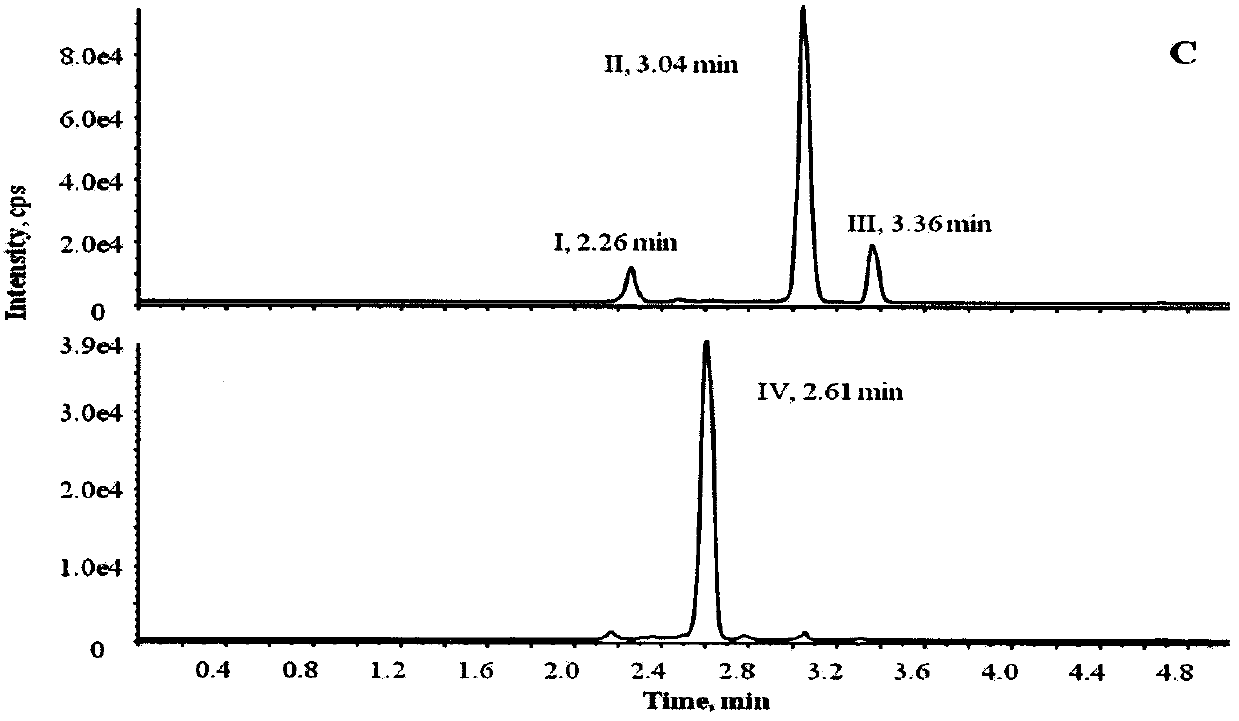
[0056] Instrument: Shimadzu LC-30AD ultra-high performance liquid chromatography (Shimadzu, Japan) in series with API5500 triple quadrupole mass spectrometer (AB SCIEX, USA), equipped with electrospray ionization source (ESI); data acquisition and processing software is Analyst1. 6.2; temperature-controlled high-speed centrifuge (Thermo Fisher, Germany); electronic analytical balance (Mettler, Switzerland);
[0057] Materials and reagents: CDP-1 standard product, batch number 1-TMH-108-1, purchased from Canada TRC Company; vancomycin, specification 1007u / mg, batch number 130360-201302, internal standard norvancomycin, purity 83.4% , batch number 130338-200303, were purchased from China National Institute for the Control of Pharmaceutical and Biological Products; acetonitrile and formic acid (chromatographically pure) were purchased from Sigma-Aldrich Company; ultrapure water was freshly prepared daily with Milli-Q; blank serum was ...
Embodiment 3
[0086] Example 3 Clinical application
[0087] Twelve hemodialysis patients (6 males and 6 females) were selected for the treatment of Gram-positive bacterial infections requiring vancomycin and TDM. Samples of trough concentrations were collected no earlier than before the second dose of administration, and 0.5- The peak concentration samples were collected in 1 hour, and the whole blood samples were centrifuged at 3000rpm for 10 minutes, and the supernatant was taken and frozen in a -70°C refrigerator for testing. The demographic characteristics and concentration data of the patients are shown in Table 9. , the ratio of CDP-1 (CDP-1-m+CDP-1-M) to vancomycin concentration is 5.9%-22.9% and 5.1%-12.2%, respectively, if there is an interaction of CDP-1 in the detection method, or Failure to separate vancomycin and CDP-1 may lead to inaccurate dose adjustment in 2 patients (16.7%), thus affecting the individualized precise drug administration of patients.
[0088] Table 9 Demog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com