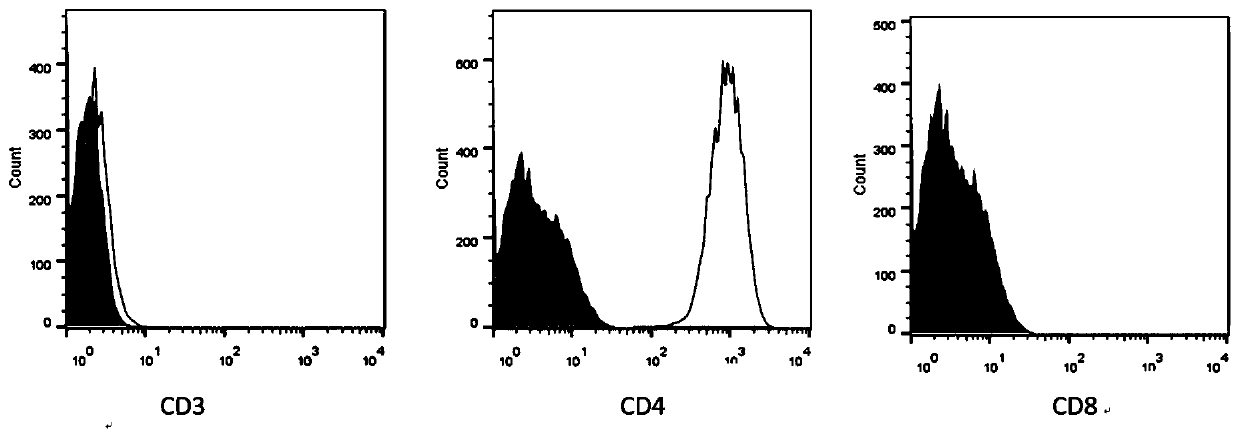
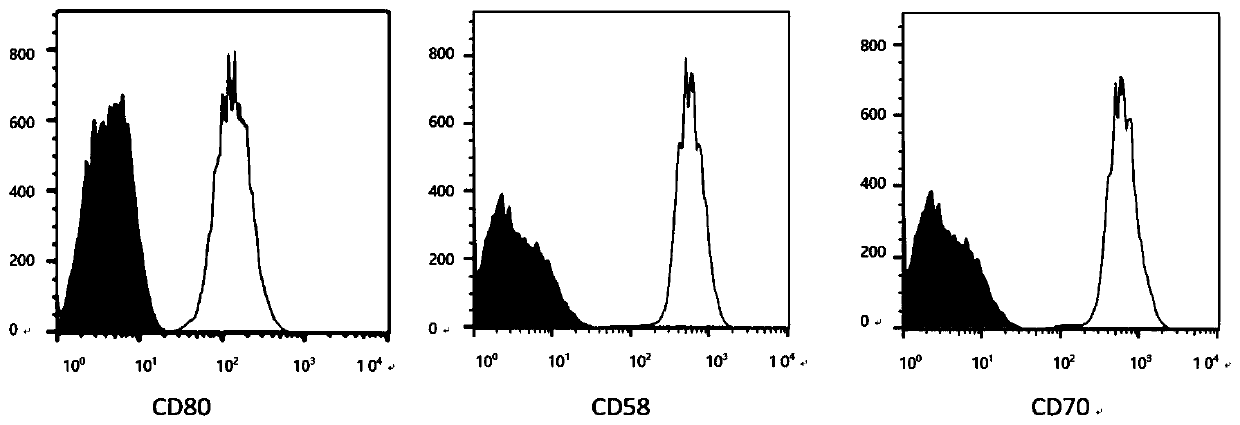
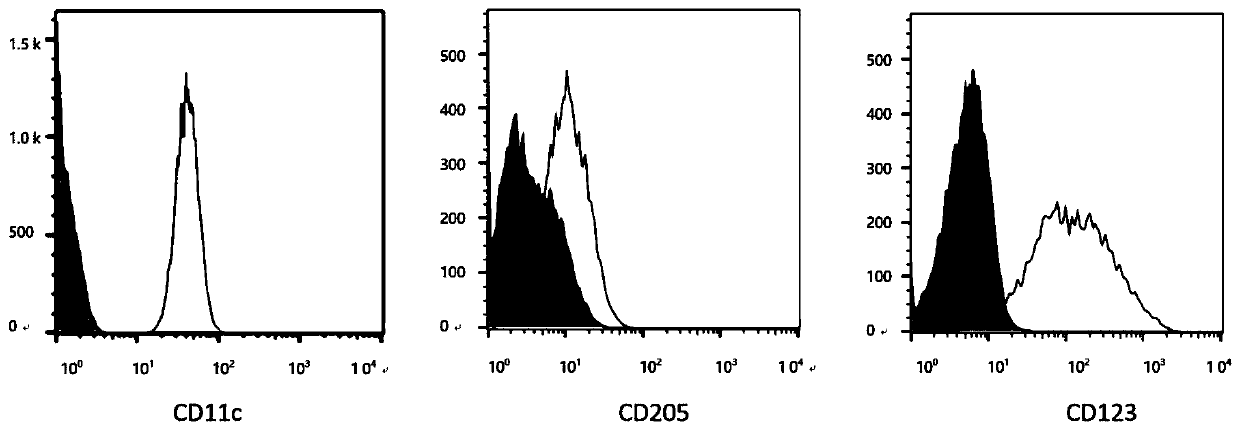
Gene, vector and method for preparing immortalized dendritic cells and immortalized dendritic cells
A dendritic cell and immortalization technology, applied in the field of genetic engineering, can solve the problems of low presentation ability and limited application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] plasmid extraction
[0053] 1) Shaking protocol: Melt Glycerol bacteria on ice, Glycerol bacteria contain the vector P-ST40-TAX2 for preparing immortalized dendritic cells (the vector contains ST40 gene and TAX2 gene), take 100 μL to 3 mL each containing 100 μg / mL In the LB medium of ampicillin, activate at 37°C and 220rpm for 6h. The activated bacterial liquids were respectively expanded into 100 mL LB medium containing 100 μg / mL ampicillin, and cultured overnight at 37° C. and 220 rpm. The next day, centrifuge at 4000×g for 10 minutes to collect the sediment of each bacterial solution.
[0054] Among them, glycerol bacteria: after transforming the competent cell Stbl3 with the vector containing ST40 gene and TAX2 gene, pick the monoclonal shaking bacteria, discard the supernatant after the bacterial solution is centrifuged, keep the bacterial sediment, add 25% glycerol to weigh the bacterial sediment Suspended to obtain glycerol bacteria. Competent cells Stbl3 were...
Embodiment 2
[0082] Isolation and Activation of Dendritic Cells from Peripheral Blood by Adherence Method (DC)
[0083] 1) Blood collection and separation of PBMC;
[0084] 2) Adjust the PBMC to 1×10 medium 1640+10% FBS 6 Cells / mL, as in Petri dish, at 37°C 5% CO 2 Incubator, rest overnight; collect suspension cells, mark as T+B cells, and set aside;
[0085] 3) Pipette the cells attached to the bottom of the culture dish with medium 1640+10% FBS+100IU / mL IL-2, and collect the adherent mononuclear cells, which are peripheral blood-derived DCs,
[0086] 4) Adding 35 μg / mL of PHA, stimulating and culturing the cells for 24 hours, and then preparing for use to obtain dendritic cells.
Embodiment 3
[0088] Construct lentiviral vector and transform DC
[0089] 1) Packaging lentivirus: 293T cell plating density: 1.8×10 7 , 20mL OPTI-MEM medium placed at 37 °C 5% CO 2 to cultivate. The amount of transfection plasmid obtained in Example 1: the amount of packaging plasmid: 15 μg, P-ST40-TAX2 plasmid (plasmid for preparing immortalized dendritic cells): 15 μg, adding the plasmid into the buffer and shaking and mixing for 5 seconds, adding the transfection Transfection reagent: 60 μL, pipette to mix 5 times, incubate at room temperature for 10 minutes, evenly distribute the transfection mixture into the cells, 37°C 5% CO 2 to cultivate. 3 hours after transfection, fresh medium was replaced, and 37 mL of OPTI-MEM (6% FBS) medium was added to each T175 culture bottle, and the supernatant was harvested at 96 hours for later use.
[0090] 2) DC infected with lentivirus: use 1mL 1640+10%FBS+0.1%polybrene+100μL virus supernatant to resuspend the activated DC obtained in Example 2,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com