Ammonium removal ultrafiltration membrane for non-photoelectric response oxidation degradation of ammonia nitrogen, preparation method and application in sewage deammonization
An oxidative degradation, photoelectric response technology, applied in the fields of oxidized water/sewage treatment, chemical instruments and methods, water/sewage treatment, etc., can solve the problems of increasing the salinity of wastewater, restricting development, increasing the amount of materials used, etc., to avoid nitrate Nitrogen and nitrous nitrogen, improve catalytic ability, avoid secondary pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
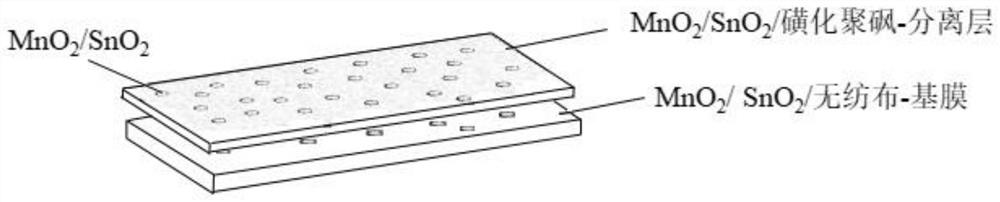
[0040] Such as figure 1 As shown, Example 1 of the present invention provides a non-photoelectric response to oxidative degradation of ammonium nitrogen deammonization ultrafiltration membrane, including a base membrane support layer and a separation layer arranged in sequence; the separation layer contains MnO 2 / SnO 2 Composites MS. The base film support layer is a non-woven fabric soaked with saturated potassium permanganate and saturated stannous chloride solution.
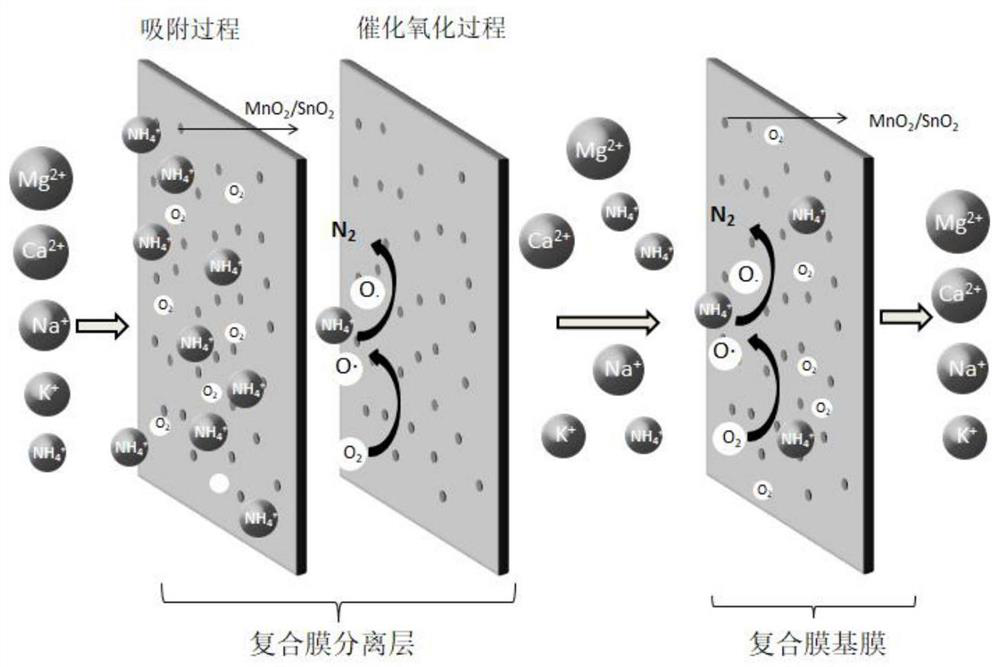
[0041] Such as figure 2 As shown, the working mechanism of the composite ammonium removal membrane is as follows: firstly, the MnO in the separation layer of the composite ammonium removal membrane is used 2 / SnO 2 The compound will dissolve oxygen and NH in the water 4 + Adsorbed to the surface of the separation layer of the composite deammonization membrane, the MnO 2 / SnO 2 Under the action of catalysis, the dissolved oxygen will be converted into a strong oxidizing intermediate product O, and the ...
Embodiment 2
[0046] Embodiment 2 of the present invention provides an ultrafiltration membrane for deammonization of ammonia nitrogen by non-photoelectric response oxidation degradation, and the ultrafiltration membrane is prepared through the following process steps.
[0047] (1)MnO 2 / SnO 2 Composite material powder synthesis method: prepare 0.5mol / L potassium permanganate and 1mol / L tin protochloride solution, measure a certain amount of potassium permanganate solution, add protochloride dropwise to the solution under stirring condition Tin, wherein the molar ratio of potassium permanganate to stannous chloride is 1:3, the formed black precipitate is filtered and washed with deionized water, and then dried in an oven at 80°C for 10 hours to obtain MnO 2 / SnO 2 The composite material (abbreviated as MS) is ground into powder for later use.
[0048] (2)MnO 2 / SnO 2 Preparation of modified non-woven base film: prepare saturated potassium permanganate and stannous chloride solution, fi...
Embodiment 3
[0051] Embodiment 3 of the present invention provides a method for preparing a non-photoelectric-responsive ammonia nitrogen oxidation deammonization ultrafiltration membrane, comprising the following steps:
[0052] Measure 500mL of 0.5mol / L potassium permanganate and 750mL of 1mol / L stannous chloride solution, add the stannous chloride solution dropwise to the potassium permanganate solution while stirring, and mix potassium permanganate and chloride in the solution The molar ratio of stannous is 1:3. During the stirring process, a black precipitate is formed. After the black precipitate is filtered and washed with deionized water for several times, it is dried in an oven at 80°C for 10 hours, and then ground into powder to obtain MnO 2 / SnO 2 Composite powder material. Take a non-woven fabric with a size of 100cm×250cm and soak it in saturated potassium permanganate and saturated stannous chloride solution in turn, soak for 1min each, after taking it out, the non-woven fab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com