Fluorescent quantitative PCR kit for detecting vancomycin-resistant vanA gene of enterotoxin
A vancomycin-resistant, fluorescence quantitative technology, applied in the field of bioengineering, can solve problems such as difficult clinical treatment, increased infection incidence and mortality, and achieve the effect of optimizing response parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: S1, extract GenBank: KF052036.1
[0054]
[0055]
[0056] S2, the following sequence is designed by software (Beacon Designer8.20):
[0057] SEQ ID NO: 1 upstream primer F: 5'-GCTACGTTTACCTATCCTG-3'
[0058] SEQ ID NO: 2 downstream primer R: 5'-CAGCCTGCTCAATTAAGA-3'
[0059] SEQ ID NO: 3TaqMan probe sequence P: 5'-FAM-TTGCTGTCATATTGTCTTGCCGATTC-BHQ-3'; the reporter fluorescent group labeled at the 5' end of the probe and the quencher group labeled at the 3' end can be selected according to the fluorescent PCR equipment, etc. The specific situation is selected separately, and the detection of fluorescent RT-PCR.
[0060] (1) Take the RT-PCR reaction solution, hot-start Taq enzyme and probe according to the number of detection samples n (n=the number of samples to be tested+2) and mix them in a centrifuge tube, vibrate on a vortex shaker, and Aliquot the tubes and cap the tubes for later use.
[0061] (2) Now add the negative control solution into an...
Embodiment 2
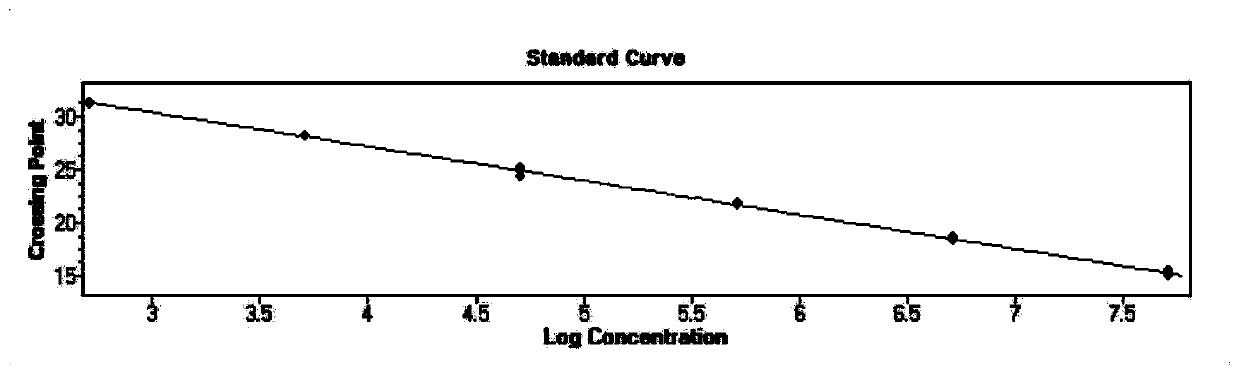
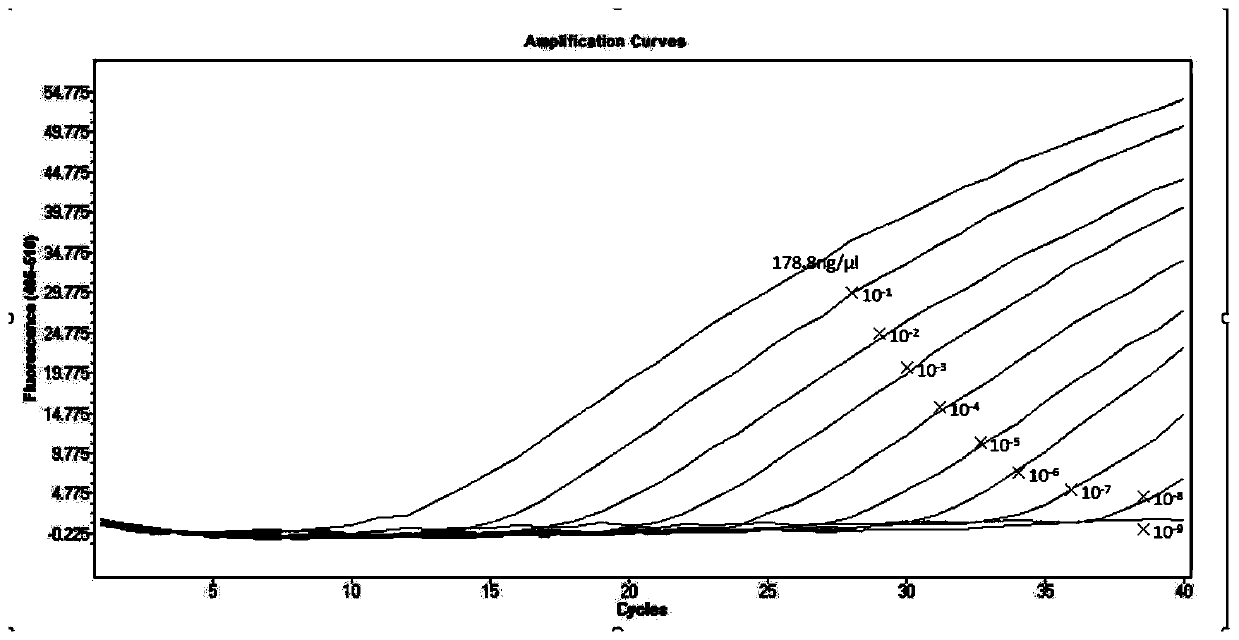
[0071] Embodiment 2: standard curve test
[0072] Take the positive plasmid in the kit, determine its concentration to be 178.8ng / μL, and carry out 10-fold gradient dilution with DEPC water, and dilute 6 gradients in total, and perform fluorescent RT-PCR detection on each dilution respectively to obtain Its slope and correlation coefficient R2 value.
[0073] Experimental results: For the standard curve of the plasmid, see figure 1 , the slopes are -3.323, respectively, according to the formula E (amplification efficiency) = 10-1 / slope, the amplification efficiency converted into a percentage ((E-1) × 100%) is 103.1%, between 90% to 110% between 0.99 and 1, indicating that the amplification efficiency of the method is good; the R2 values are 0.999, respectively, between 0.99 and 1, indicating that the method has high reliability.
Embodiment 3
[0074] Embodiment 3: specificity test
[0075] Take Escherichia coli standard strain, Salmonella standard strain, Staphylococcus aureus standard strain, Campylobacter standard strain DNA each 2 μL as the template for the specificity detection of fluorescent RT-PCR method, and set the plasmid as positive control and blank plasmid as negative As a control, DEPC water was used as a blank control group.
[0076] RT-PCR amplification conditions: 5 min at 42°C, pre-denaturation at 95°C for 10 s, cycled 40 times according to the following parameters: denaturation at 94°C for 15 s, fluorescence collection at 59°C for 30 s. Fluorescence detection at 59°C, detection channel: FAM.
[0077] The results of fluorescent RT-PCR showed that enterotoxins containing vanA gene amplified curves, while other bacteria did not have S-type amplification curves. At the same time, DEPC water blank control and blank plasmid negative control were both established, indicating that the method has good spec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com