Fluorescence derivation reagent for detecting aldehyde substances and preparation method and application thereof
A technology of derivatizing reagents and substances, which is applied in the field of fluorescent derivatizing reagents for detecting aldehydes and its preparation, which can solve the problems of poor stability, high polarity, lack of chromophores or fluorophores, and achieve low detection limits, Simple operation and high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
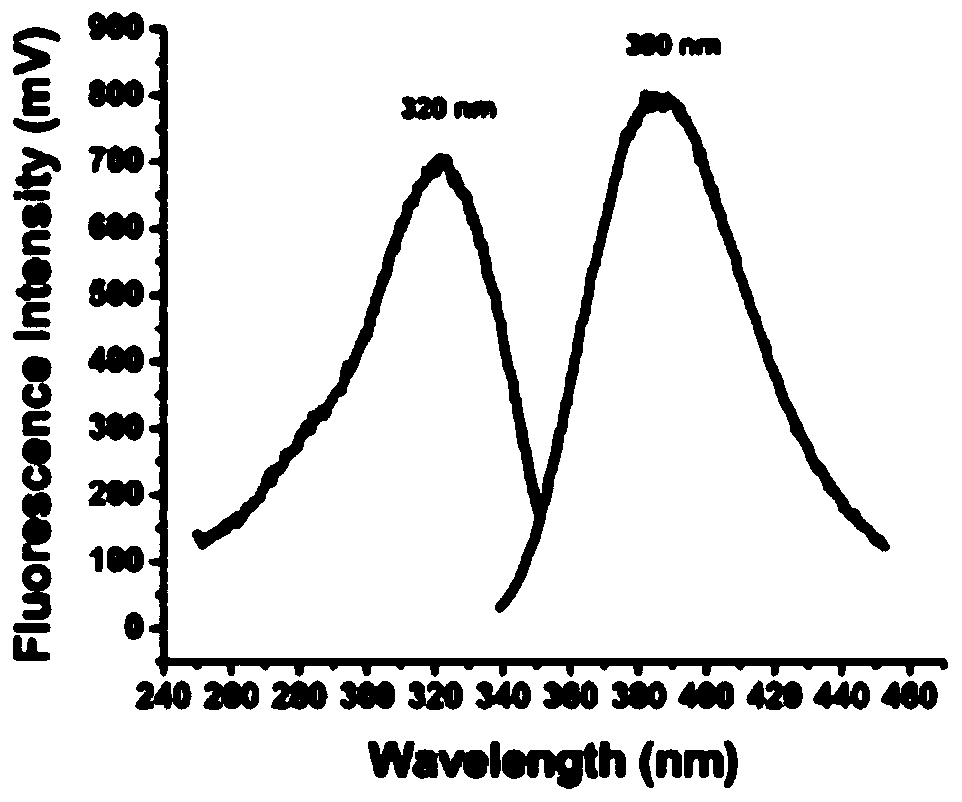
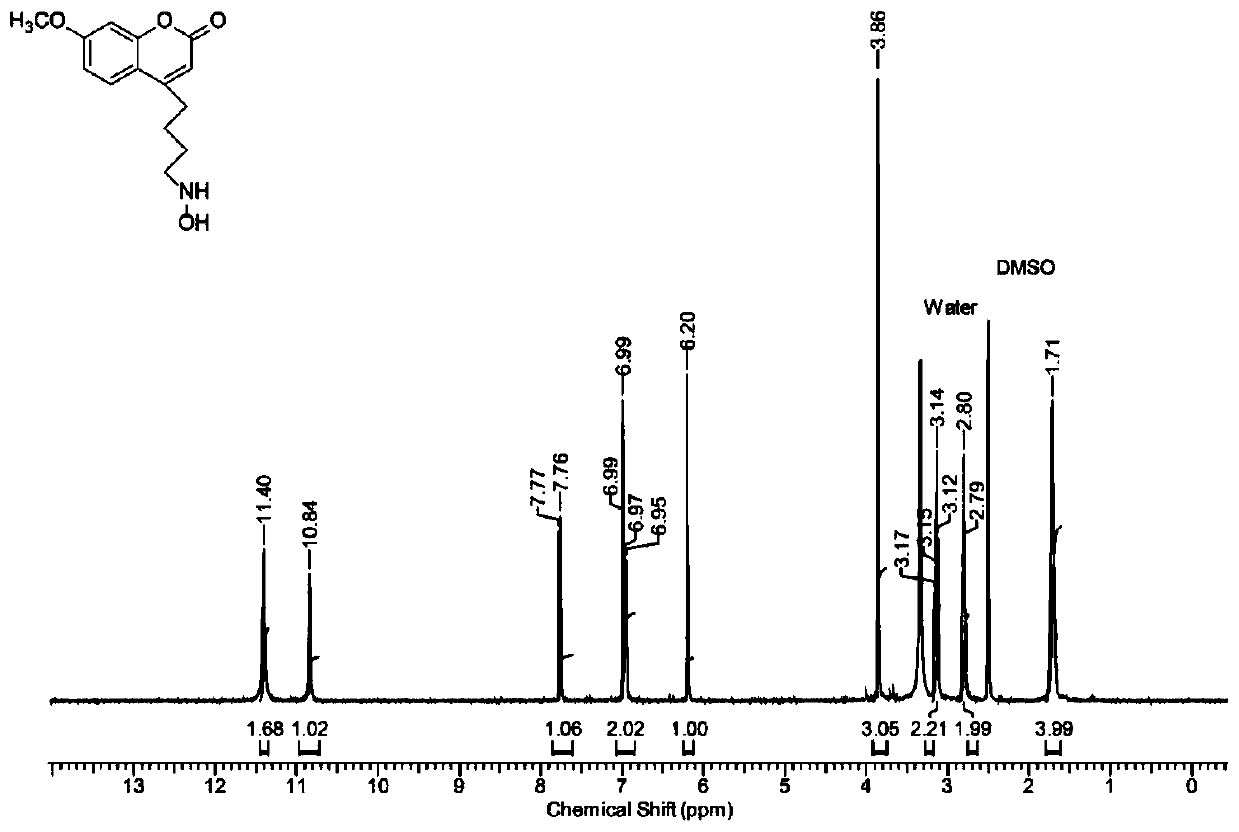
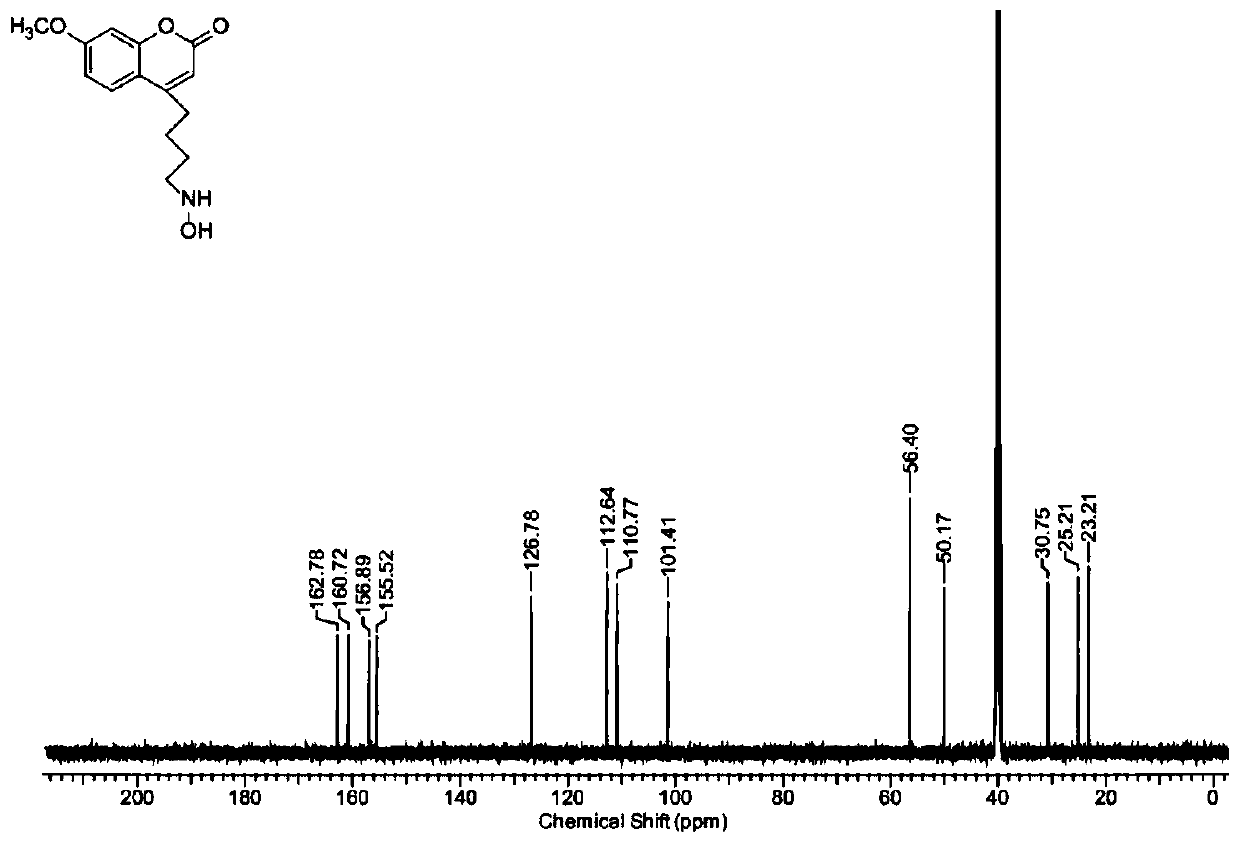
[0042] Example 1 Preparation and characterization of 4-hydroxyaminobutyl-7-methylamino-coumarin
[0043] 1. Preparation of 4-hydroxyaminobutyl-7-methylamino-coumarin
[0044] 1. Synthesis of Ethyl 2-(1-hydroxycyclopentyl) acetate (compound 6)
[0045]
[0046]TMSCl (0.9mL, 72mmol) and zinc powder (6.3g, 96mmol) were added to 150mL of anhydrous ether, and stirred at room temperature for 15min. Heat to reflux (40°C), add dropwise a mixed solution of cyclopentanone (5g, 60mmol) and ethyl bromoacetate (12.0g, 72mmol) in the above reflux system, after the addition is complete, reflux for 20min, and then continue for 1h , TLC monitoring until the disappearance of the starting material. After the reaction was completed, after the reaction system was cooled, 300 mL of 2N hydrochloric acid was added and stirred for 15 min. Separate the organic phase, extract the aqueous phase with diethyl ether (2×50 mL), combine the organic phases, and wash with 5% NaHCO 3 solution to wash the ...
Embodiment 2
[0083] Embodiment 2 is used to detect the kit and detection method of aldehydes
[0084] 1. Composition of the detection kit
[0085] (1) Standard samples of aldehyde compounds: include the following components (by molar concentration)
[0086] 2mL formaldehyde standard concentration sample (1mmol / L) in each bottle.
[0087] 2mL acetaldehyde standard concentration sample (1mmol / L) in each bottle.
[0088] 2mL standard concentration sample of propionaldehyde (1mmol / L) in each bottle.
[0089] 2mL standard concentration sample of butyraldehyde (1mmol / L) in each bottle.
[0090] 2mL standard concentration of valeraldehyde in each bottle (1mmol / L).
[0091] 2mL standard concentration sample of hexanal (1mmol / L) in each bottle.
[0092] (2) 2mL HAMC aldehyde detection reagent (0.5mmol / L) per bottle.
[0093] (3) The detection buffer solution includes the following components (in terms of molar concentration): 2 mL of acetic acid-sodium acetate buffer solution (0.1 mol / L, pH 4...
Embodiment 3
[0097] The performance measurement of embodiment 3 kits
[0098] 1. Linear regression equation: select a series of C1-C6 fatty aldehyde standard solutions with different concentrations (from LOD to 8000nM) for analysis, take the concentration of the sample before derivatization as the abscissa (X), and the peak area of the derivative product as the ordinate (Y ) to get the standard curve.
[0099] 2. Detection limit: continuously reduce the concentration of reactants until the product peak S / N=3, the corresponding fatty aldehyde concentration is the detection limit.
[0100] 3. Precision: Do 6 identical reaction samples in parallel (on the same day) and inject them separately to evaluate the RSD within the day; do 6 identical reaction samples in parallel every day for three consecutive days and inject samples separately to evaluate the daily RSD. Between RSDs.
[0101] The linear range, regression equation, detection limit and precision results of formaldehyde, acetaldehyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com