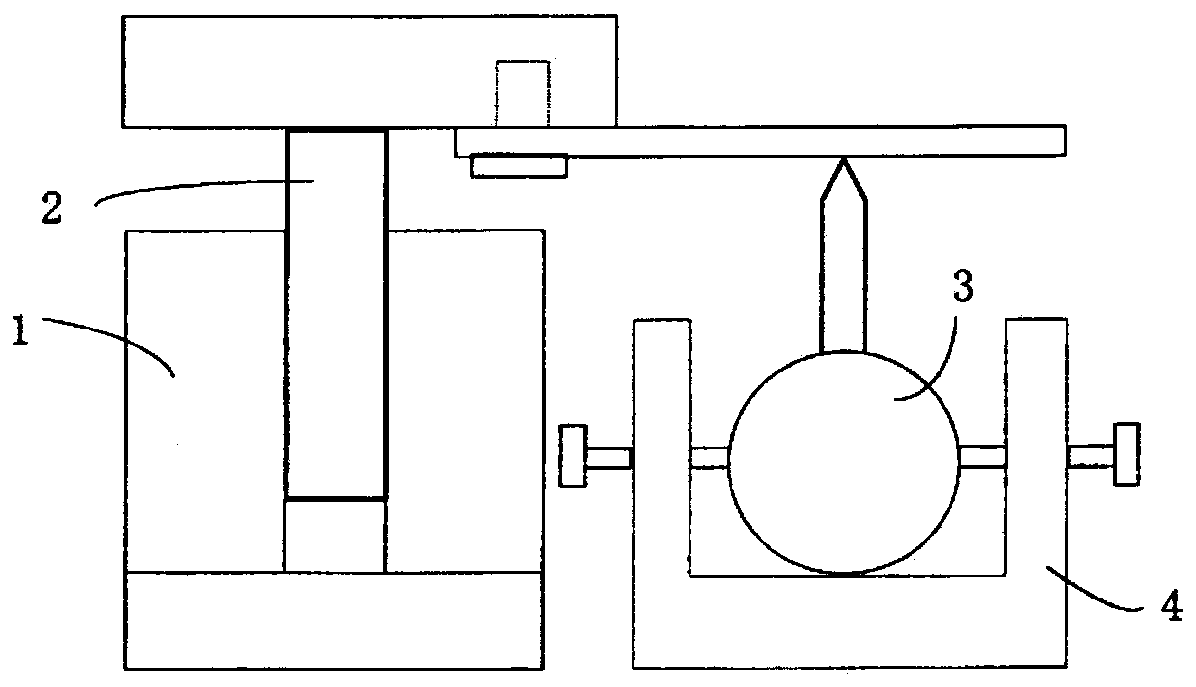
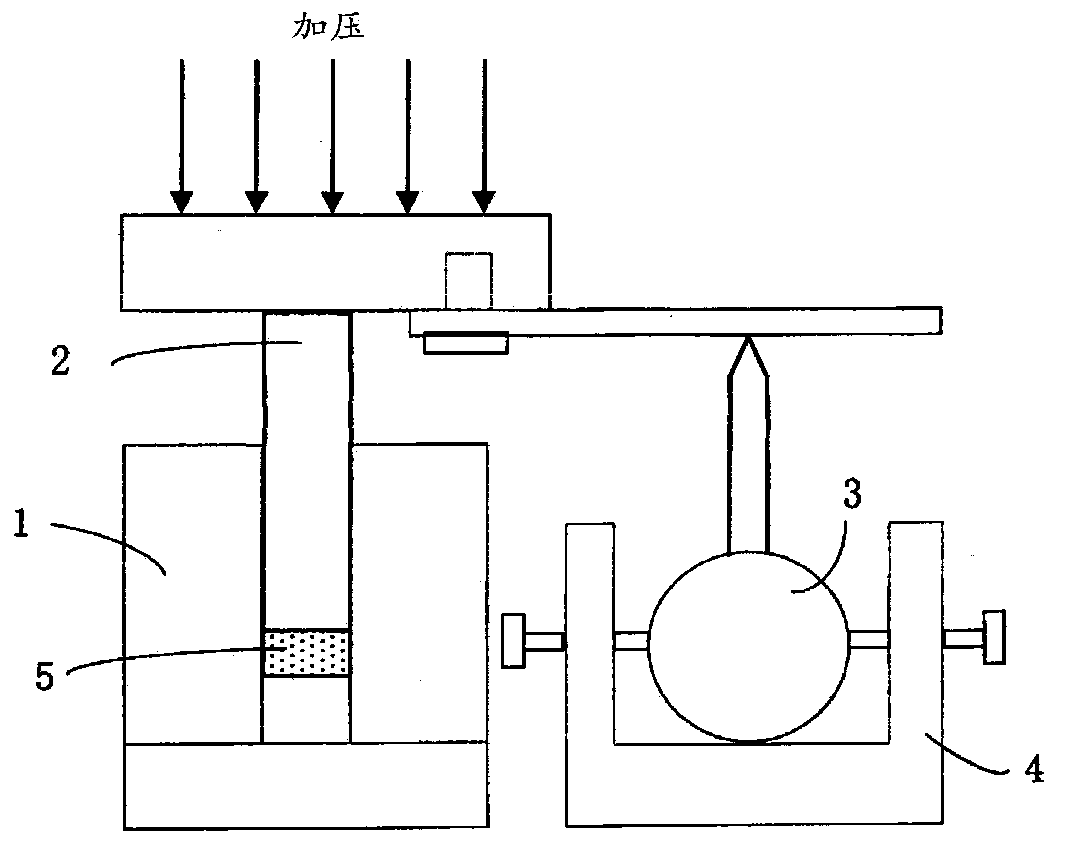
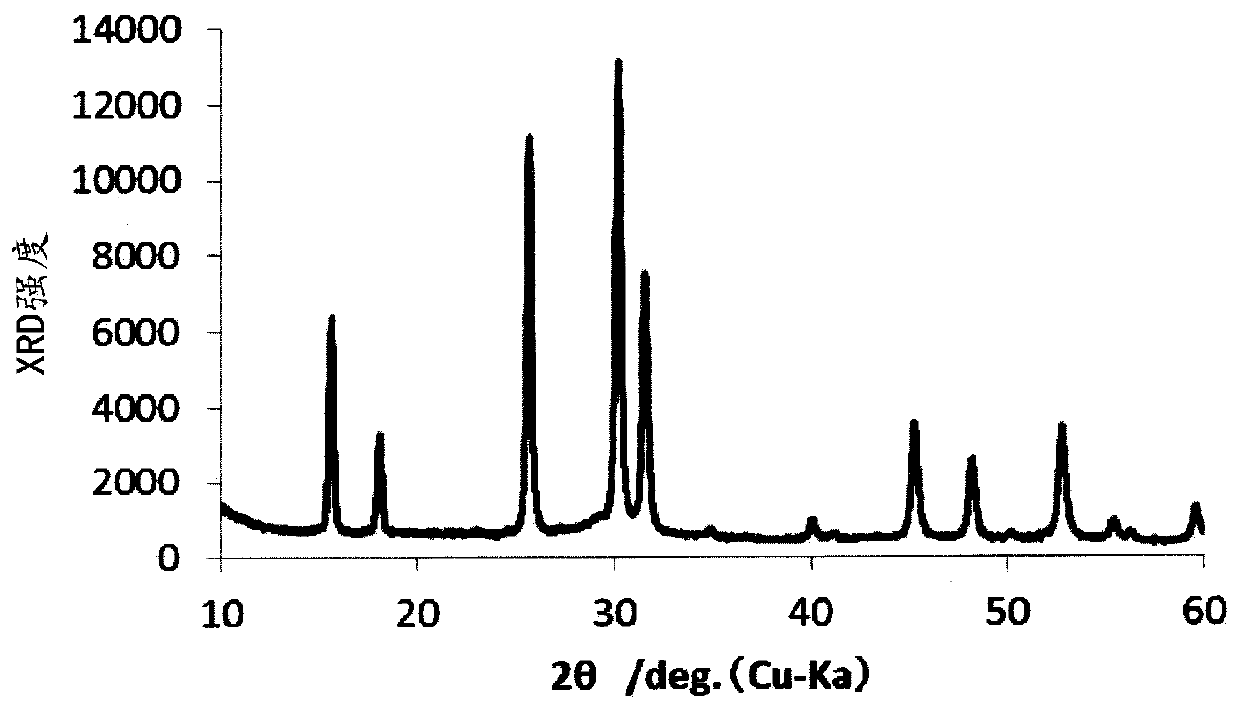
Sulfide solid electrolyte
A technology of solid electrolytes and sulfides, applied in solid electrolytes, sulfide conductors, non-aqueous electrolytes, etc., can solve the problems of smaller contact area, destruction of active material particles, and insufficient formation of ion paths, etc., and achieve high capacitance maintenance rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0159] Hereinafter, the present invention will be described in more detail based on examples.
[0160] In addition, the evaluation method is as follows.
[0161] (1) Volume reference average particle size (D 50 )
[0162] Measurement was performed using a laser diffraction / scattering particle size distribution measuring device (LA-950V2 model LA-950W2 manufactured by Horiba, Ltd.).
[0163] A mixture of dehydration-treated toluene (manufactured by Wako Pure Chemical Industries, special grade) and t-butanol (manufactured by Wako Pure Chemical Industries, special grade) at a weight ratio of 93.8:6.2 was used as a dispersion medium. After injecting 50 mL of the dispersion medium into the flow cell of the device and circulating it, the object to be measured was added, subjected to ultrasonic treatment, and then the particle size distribution was measured. In addition, the addition amount of the measurement object is based on the measurement screen specified by the device, the r...
manufacture example 1
[0238] (lithium sulfide (Li 2 S) Manufacture)
[0239] Into a 500 mL separable flask equipped with a stirrer, 200 g of anhydrous LiOH (manufactured by Honjo Chemical Co., Ltd.) dried under an inert gas was charged. The temperature was raised under nitrogen flow and the internal temperature was maintained at 200°C. Nitrogen gas was switched to hydrogen sulfide gas (Sumitomo Seika Chemicals), and lithium hydroxide anhydrate (LiOH) was reacted with hydrogen sulfide at a flow rate of 500 mL / min.
[0240] The water produced by the reaction is condensed and recovered by a condenser. 144 ml of water was recovered at the time point after allowing the reaction to proceed for 6 hours. Further, the reaction was continued for 3 hours, but generation of water was not observed.
[0241] The product powder was recovered, and its purity and XRD were measured. As a result, the purity was 98.5%, and Li 2 The peak spectrum of S.
Embodiment 1
[0243] (1) Manufacture of sulfide solid electrolyte (precursor) with argentite-type crystal structure
[0244] Li that will be produced in Production Example 1 2 S, phosphorus pentasulfide (P 2 S5 : FS SPEC, manufactured by Thermphos, with a purity of 99.9% or more) and lithium chloride (LiCl: manufactured by Sigma-Aldrich, with a purity of 99%) in molar ratio (Li 2 S:P 2 S 5 : LiCl) in a ratio of 19:5:16 to prepare a mixture of starting materials (in all examples below, the purity of each starting material is the same). 200 g of the mixture and 1780 g of zirconia balls with a diameter of 20 mm were placed in a SUS container (capacity 6 L). The container was installed in a vibrating mill (Central Chemical Machinery MD-3), and by applying mechanical energy (mechanical grinding treatment), a white-yellow powder glass (intermediate body) was obtained. The treatment conditions of the vibratory mill were set at a temperature of 40° C. at 1500 revolutions / second for 120 hours u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com