Fluorescent quantitative PCR kit for detecting rubella viruses
A rubella virus and fluorescent quantitative technology, which is applied in the determination/inspection of microorganisms, DNA/RNA fragments, recombinant DNA technology, etc., can solve problems such as non-coverage, missed diagnosis, and false positive test results, so as to improve reliability and prevent false positives. Negative, easy-to-storage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
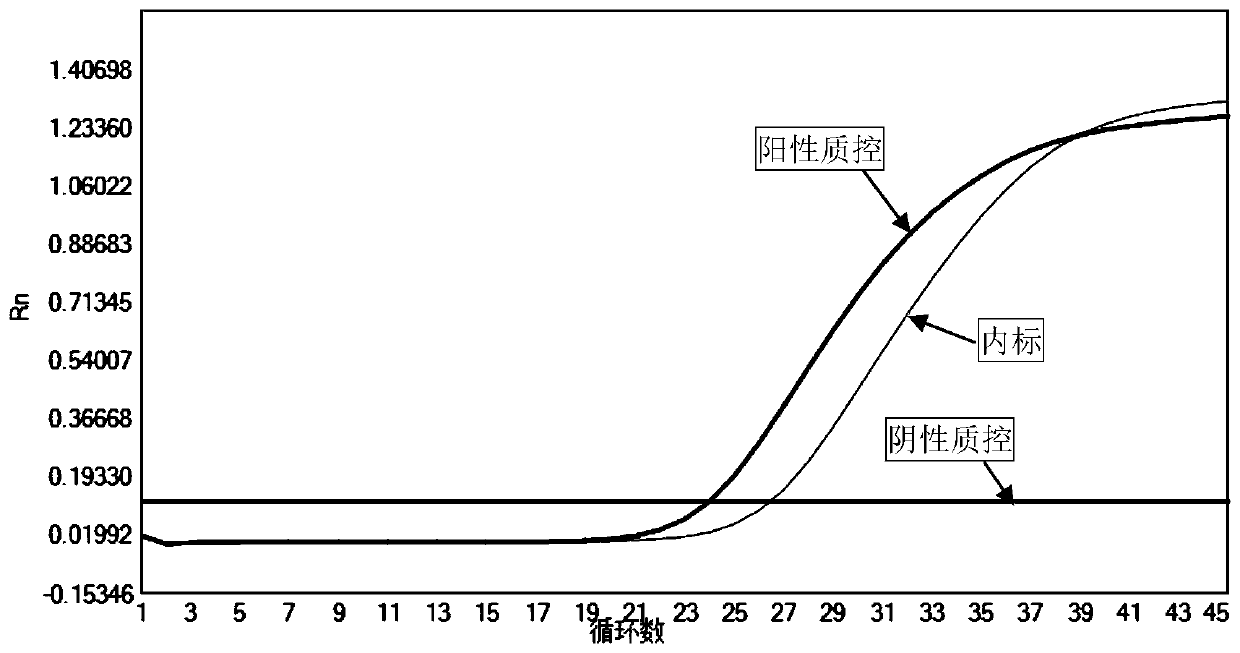
[0037] This embodiment provides a fluorescent quantitative PCR kit for detecting rubella virus, which includes a PCR reaction solution, an internal standard, a positive quality control product and a negative quality control product.
[0038] The PCR reaction solution includes upstream primer 1, downstream primer 1 and fluorescent probe 1 for detecting rubella virus, upstream primer 2, downstream primer 2 and fluorescent probe 2 for detecting internal standard, reverse transcriptase, RNase inhibitor, Taq DNA polymerase, UNG enzyme, dUTP and dNTPs.
[0039] The nucleotide sequence of the upstream primer 1 is 5'-TGCAGATGGCGCCCAGAGTGAG-3' (SEQ ID NO.1), and the nucleotide sequence of the downstream primer 1 is 5'-ACCGGCGTGGTGTATGGCACAC-3' (SEQ ID NO.2);
[0040] The base sequence of fluorescent probe 1 is 5'-TGGGCAGCAGCCCACTCCGCCC-3' (SEQ ID NO.3), its 5' end is marked with a fluorescent group FAM, and its 3' end is marked with a quenching group capable of quenching the fluorescen...
Embodiment 2
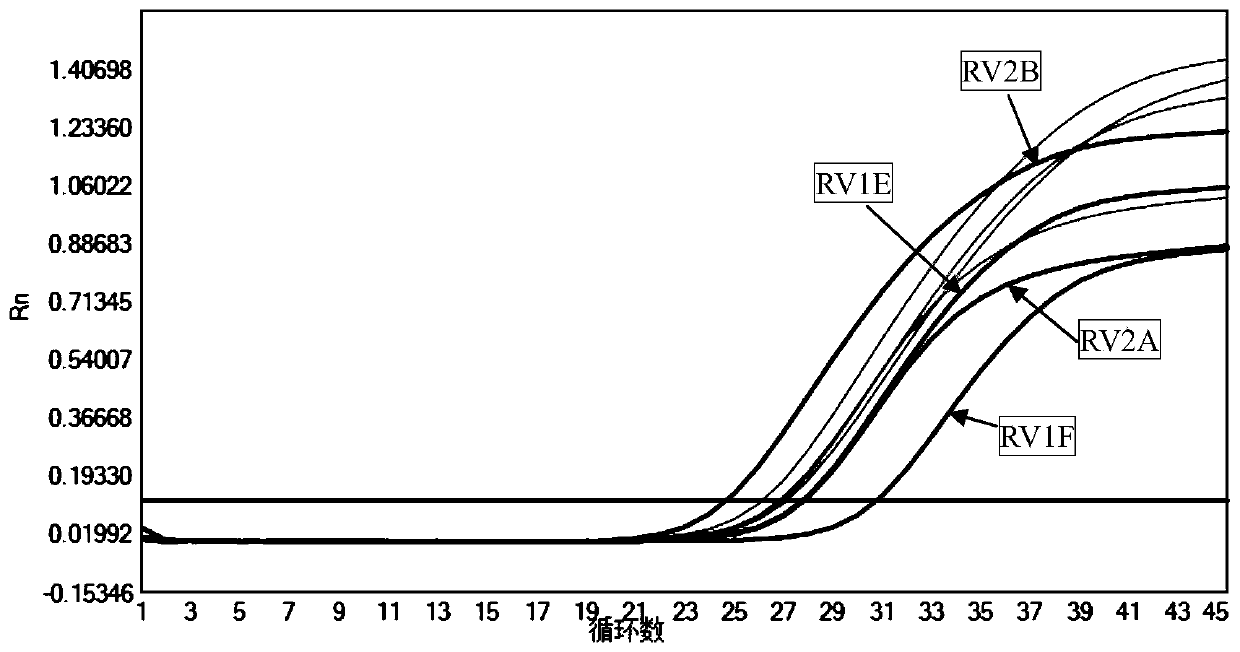
[0047] In this example, the kit described in Example 1 is used to detect the rubella virus RNA in the sample. The sample to be tested can be a sample in any form of whole blood, serum, plasma or amniotic fluid. The specific operation steps are as follows:
[0048] S1. Extract sample RNA
[0049] Aspirate 200 μL of the sample to be tested and add 5 μL of internal standard for nucleic acid extraction; take 25 μL of positive quality control and negative quality control, add 5 μL of internal standard respectively, and add physiological saline to 200 μL for nucleic acid extraction.
[0050] The virus nucleic acid extraction kit of Baitai Genomics (Ehan Jibei No. 20170246) was used to extract the nucleic acids in the samples to be tested (including internal standards), positive quality controls (including internal standards) and negative quality controls (including internal standards), For the specific operation method, please refer to the instruction manual of the kit.
[0051] S2...
Embodiment 3
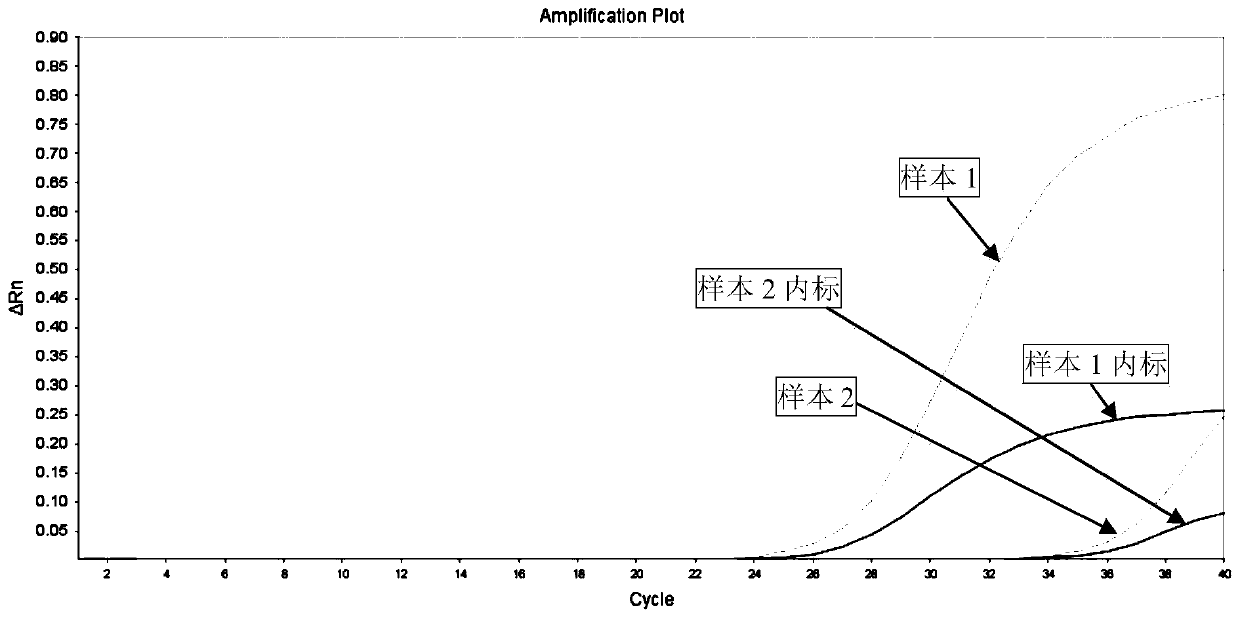
[0067] Select an RV-positive serum sample with a clinically known concentration and divide it into 2 equal parts, one of which is added with RV-negative normal serum numbered as sample 1, and the other is added with RV-negative severe hemolysis (hemoglobin concentration greater than 5.0g / L ) serum number is sample 2, and the silica gel membrane method is used to extract its nucleic acid respectively as a template, and the primers and probes designed by the present invention are used to carry out real-time fluorescent PCR reaction, and the amplification results are shown in image 3 .
[0068] like image 3 As shown, the amplification Ct values of sample 1 and its internal standard are within the specified range, and the amplification curve is good, but the amplification Ct value of sample 2 is obviously delayed compared with that of sample 1, and the amplification Ct value of sample 2 internal standard exceeds If the specified range is exceeded, the amplification curve does...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com