Fluorescent probe for detecting peroxynitrite ions, preparation method and applications thereof
A technology of peroxynitrite and fluorescent probes, which is applied in the field of analytical chemistry, can solve the problems of difficult measurement of peroxynitrite ions, short lifetime of peroxynitrite ions, and low steady-state concentration, and achieve good fluorescence The effect of emission spectrum characteristics, easy promotion, and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Synthesis of Fluorescent Probes
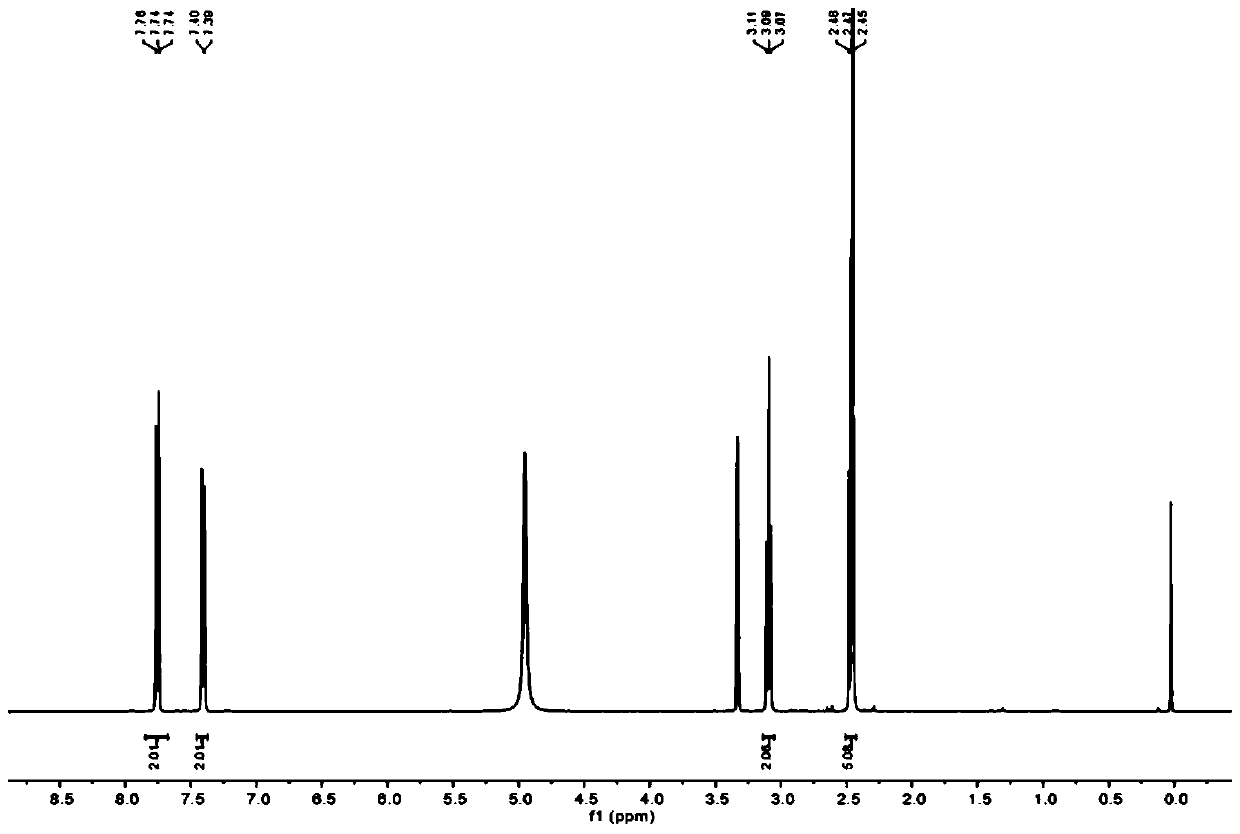
[0035] (1) Add β-alanine (600 mg, 6.7 mmol) to distilled water (4 mL), add NaOH aqueous solution (2 M, 3.4 mL), then add p-toluenesulfonyl chloride (1.83 g, 9.6 mmol), and react The mixture was stirred at 35°C. Add 1M NaOH aqueous solution to keep the pH value at 9. After the alkali is completely consumed, stir at 35°C for one hour, filter to remove unreacted p-toluenesulfonyl chloride, and use 5 M HCl aqueous solution at 0°C Acidify the reaction mixture to make pH=2, and the precipitated white precipitate is filtered, washed with cold water, dissolved in dichloromethane solvent, and purified by column chromatography (petroleum ether:ethyl acetate=5:1), which is compound 1. 1 H NMR spectrum such as figure 1 :
[0036] ;
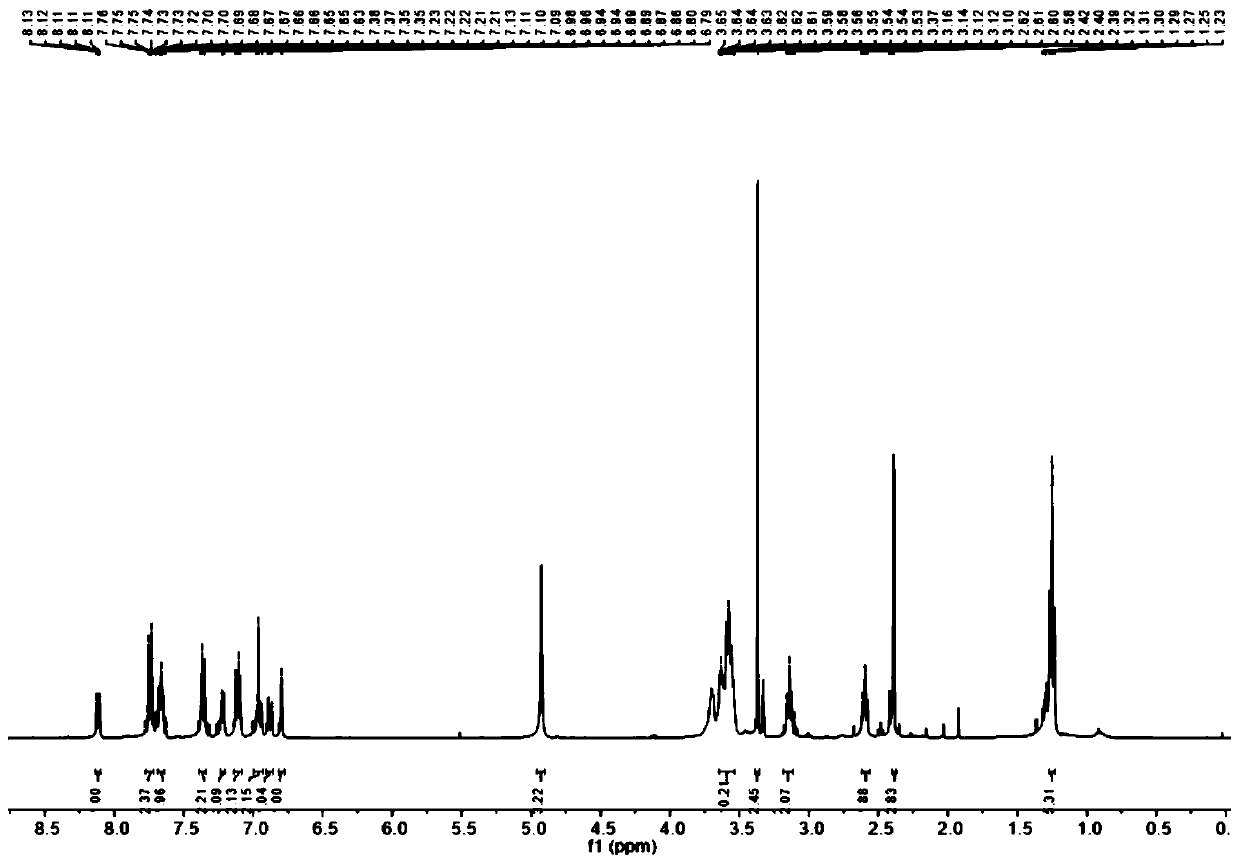
[0037] (2) Dissolve 4-diethylaminoketoacid (940 mg, 3 mmol), m-hydroxyphenylpiperazine (534.7 mg, 3 mmol) in 5 mL CH 3 In COOF, heated at reflux at 90 °C for 12 h, the reaction mixture was concentrated...
Embodiment 2
[0043] Example 2 Responses of fluorescent probes to different concentrations of peroxynitrite ions
[0044] Prepare the fluorescent probe obtained in Example 1 as a mother solution (containing 10% acetonitrile), add the same volume of solutions containing peroxynitrite ions at different concentrations, and use water as a blank (0 μM) to make the probe concentration 5 μM, for fluorescence detection (λ ex=540nm); Calculate the fluorescence intensity in each system; Evaluate the response performance of the probe to peroxynitrite ion, such as Figure 4 (A) shows: the probe can respond to peroxynitrite ion at a concentration of 5-200 μM; analyze the linear relationship between the fluorescence intensity at 581 nm and the concentration of peroxynitrite ion at 5-50 μM, as shown in Figure 4 (B) As shown: within this concentration range, the linear relationship between fluorescence intensity and peroxynitrite ion concentration is good, and the linear regression equation is y=24.34x-4...
Embodiment 3
[0045] Example 3 Response time of fluorescent probes to peroxynitrite ions
[0046] Prepare the fluorescent probe obtained in Example 1 as a mother solution (containing 10% acetonitrile), and add a solution containing peroxynitrite ions so that the concentration of the probe is 5 μM, and the concentration of peroxynitrite ions is 100 μM , fluorescence detection is performed every 5s (λ ex =540 nm) for a total of 10 min; calculate the fluorescence intensity in each system; evaluate the response speed of the probe to peroxynitrite ion, as Figure 5 Shown: join ONOO - After that, the response time is less than 5 s, indicating that the probe is ONOO - Very responsive.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com