Traditional Chinese medicine composition for treating abnormal fat metabolism diseases and application thereof
A fat metabolism abnormality and composition technology, applied in metabolic diseases, drug combinations, medical preparations containing active ingredients, etc., can solve problems restricting the clinical promotion and application of Kuhuang Granules, and improve biochemical indicators and imaging evaluation Result, good curative effect, effect of expanding clinical indications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
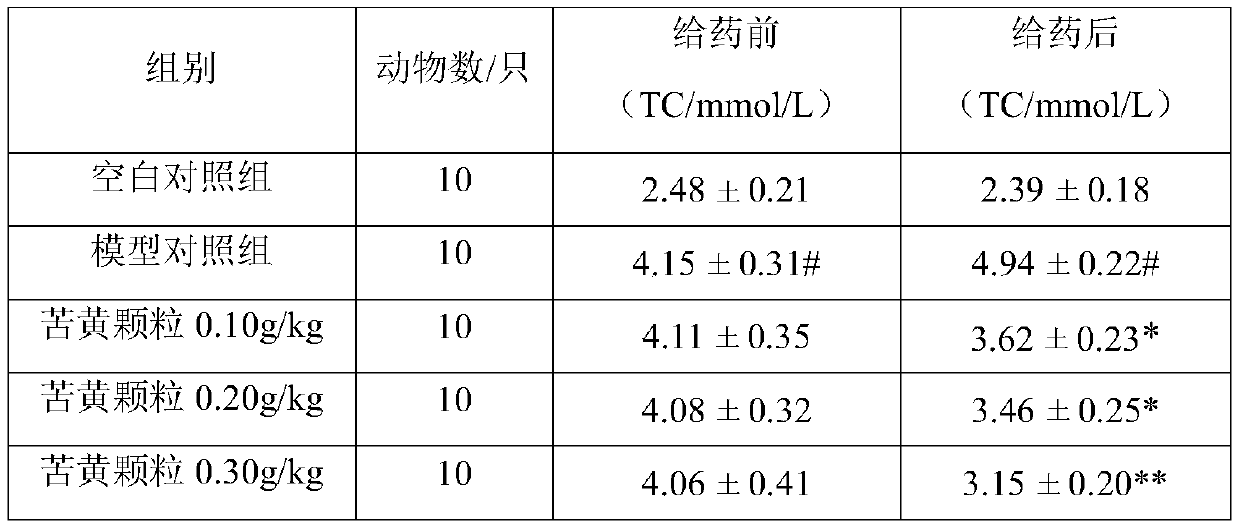
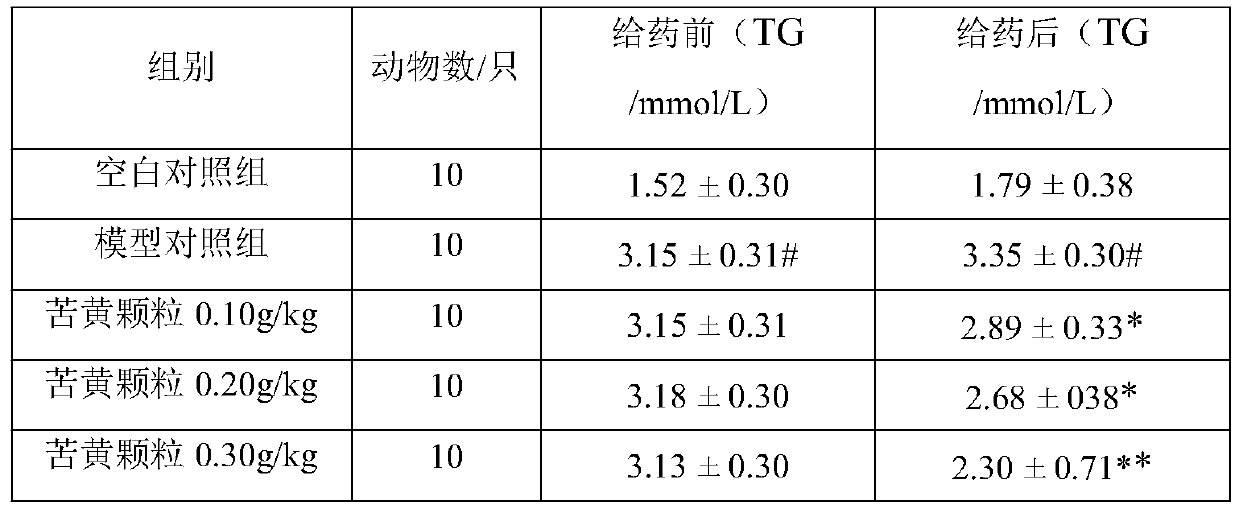
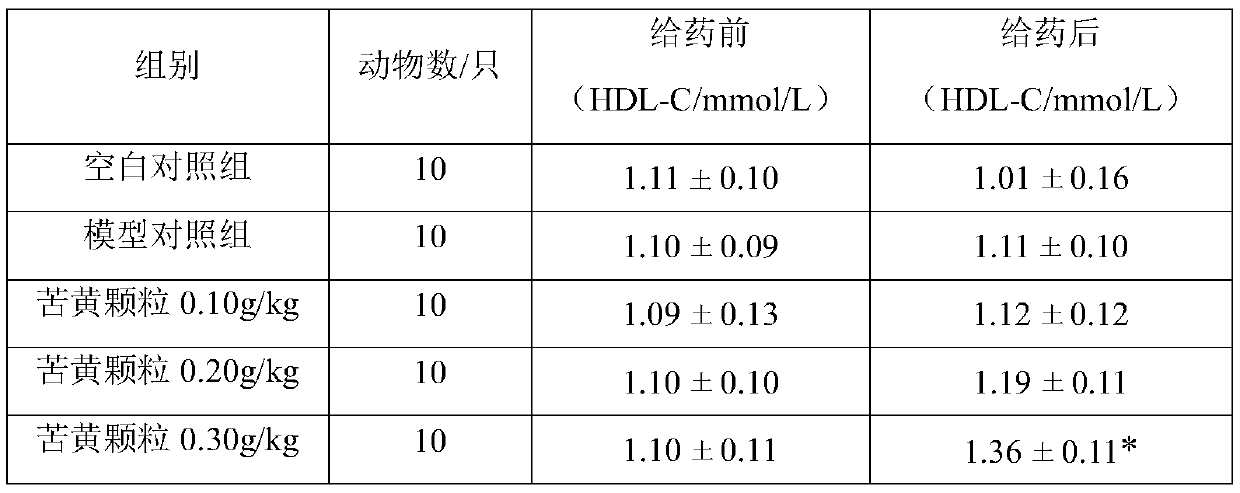
[0020] Example 1 Observation of curative effect of Kuhuang granule on hypolipidemic effect of hyperlipidemia model rats.
[0021] 1 Materials and methods
[0022] 1.1 Experimental drug: Kuhuang Granules, produced by Leiyunshang Pharmaceutical Group Co., Ltd., approval number: Z20030141; Chinese medicine raw materials: 833.3 parts of Yinchen, 833.3 parts of Spring Bupleurum, 266.7 parts of Sophora flavescens, rhubarb 333.3 copies, 625 copies of Daqingye.
[0023] 1.2 Dosage: the recommended dose of the composition tablet of the present invention is adult (by 60kg body weight > 18g per day, which is equivalent to 0.3g / kg / d. Oral administration once a day, after continuous gavage for 28d, measure each index. Rat gavage volume is 0.5mL / 100g.Set up blank control group (0g / kg), model control group (0g / kg) simultaneously, replace test substance with sterile water, each time gavage volume and each test substance Groups are the same.
[0024] 1.3 Instruments and reagents
[0025] 1...
Embodiment 2
[0065] Embodiment 2 Improves the clinical observation of fatty liver and body weight
[0066] 1. Case selection, according to the non-alcoholic fatty liver diagnostic criteria in the guidelines for the diagnosis and treatment of alcoholic fatty liver disease formulated by the non-fatty liver and alcoholic liver disease group of the hepatology branch of the Chinese Medical Association in 2010
[0067] 1) No history of drinking or alcohol consumption equivalent to less than 140 grams / week (less than 70 grams / week for women);
[0068] 2) Obesity, diabetes, hyperlipidemia, etc. with risk factors;
[0069] 3) In addition to the clinical manifestations of the primary disease, there are non-specific symptoms and signs such as fatigue, indigestion, dull pain in the liver area, hepatosplenomegaly;
[0070] 4) Serum transaminase and glutamyl transpeptidase levels may increase from mild to moderate, with alanine aminotransferase as the main increase;
[0071] 5) Liver imaging findings ...
Embodiment 3
[0088] And through the liver imaging diagnosis after treatment, the features of fatty liver in 40 patients disappeared or were significantly alleviated, and the total effective rate of treatment was 85%. The test population has no obvious discomfort, indicating that the present invention has a good effect of improving fatty liver and reducing body weight. Example 3 Experimental Research on Resistance to Chemical Liver Injury
[0089] 1. Experimental materials and drugs
[0090] 1. Drugs and reagents
[0091] AST, ALT, MDA, SOD kits and Coomassie brilliant blue protein kits were purchased from Nanjing Jiancheng Bioengineering Institute; carbon tetrachloride (CCl 4 , analytically pure, Shanghai Lingfeng Chemical Reagent Co., Ltd.), use peanut oil to prepare 0.1% peanut oil solution; galactosaminoside (D-GalN, Sigma company), distilled water before use and diluted to 70mg / mL solution; Bifendate tablets (Jiangsu Pengyao Pharmaceutical Co., Ltd.), grind into fine powder and add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com