Method for determining rivaroxaban and related substances thereof by using high performance liquid chromatography
A high-performance liquid chromatography and rivaroxaban technology is applied in the field of simultaneous determination of rivaroxaban and related substances by high-performance liquid chromatography, and can solve the problems of low detection sensitivity, poor detection method durability, chromatographic column damage and the like , to achieve the effect of simple data processing, good separation and detection, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
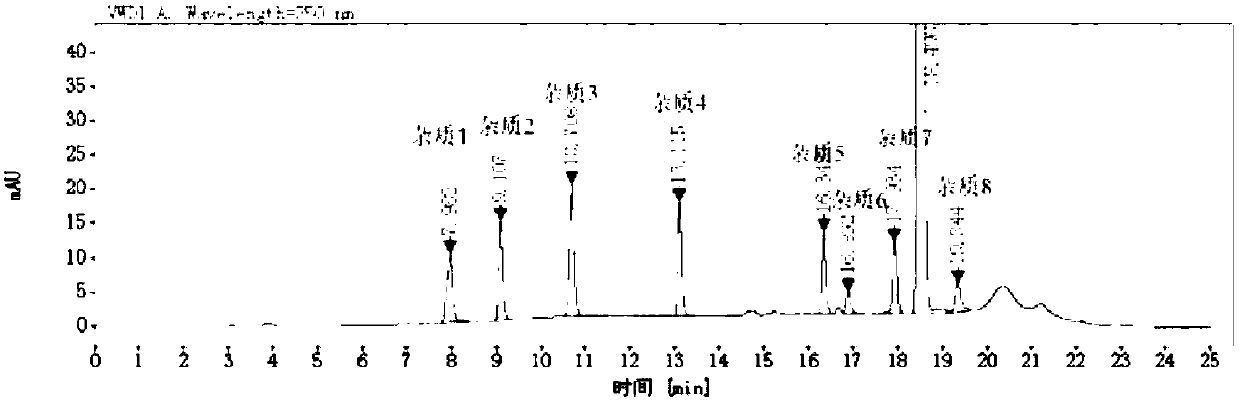
[0072] Embodiment 1 Determination of rivaroxaban content and its related substances using the method of the present invention
[0073] 1.1 Instruments and conditions:
[0074] Agilent 1260 liquid chromatography system, column: Yuexu LP-C18 (250mm*4.6mm, 5μm), the detection wavelength is 220nm, 250nm, the column temperature is 35°C, and the flow rate is 1.0mL / min;
[0075] The trapping column is Shimadzu's Ghost Trap DS.
[0076] 1.2 Test steps
[0077] 1) Preparation of mobile phase A: Take 1.42 g of sodium dihydrogen phosphate, dissolve it in 1000 mL of water, adjust the pH to 6.0 with phosphoric acid, and use it as mobile phase A; mobile phase B is acetonitrile;
[0078] 2) Prepare the reference substance solution of rivaroxaban and its related substances:
[0079] ① Preparation of mixed solvent:
[0080] Use mobile phase A and mobile phase B to prepare mixed solvent, wherein the volume ratio of mobile phase A and mobile phase B is 50:50;
[0081] ② Preparation of r...
Embodiment 2
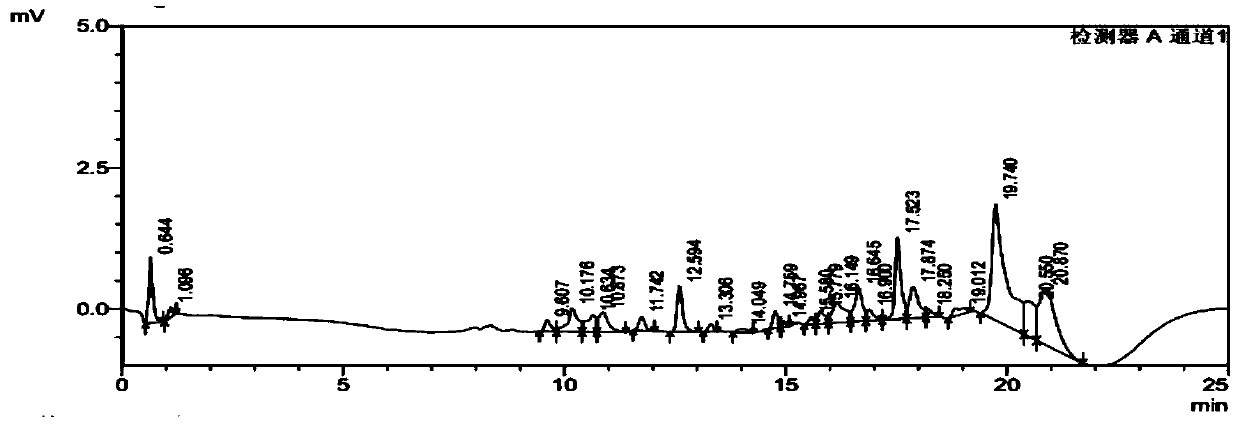
[0095] Example 2 Comparative example of prior art detection of rivaroxaban and its impurities
[0096] Using the chromatographic column and detection method described in Example 1 of patent CN105004802B to detect the content of rivaroxaban and the above-mentioned 8 related substances;
[0097] 1) Preparation of mobile phase A: Weigh 2.0 g of sodium pentanesulfonate, pipette 2.0 mL of phosphoric acid into a 1000 mL volumetric flask, add water to dissolve and dilute to the mark, and adjust the pH to 4.0 with 1 mol / L sodium hydroxide solution; Mobile phase B is acetonitrile;
[0098] 2) Take 25 mg of rivaroxaban, add an appropriate amount of related substance reference substance stock solution prepared in Example 1 of the present invention, and dissolve the sample with 30% acetonitrile aqueous solution to obtain a sample solution;
[0099] 3) Take 10 μl of each sample solution of the blank control and step 2) and inject into the Shimadzu LC-20AT chromatograph. The temperature...
Embodiment 3
[0104] Embodiment 3 The impact of different mobile phase pH values on analysis and detection
[0105] Dissolve sodium dihydrogen phosphate in water with reference to the method in Example 1, adjust the pH to 6.0, 5.5, and 6.5 with phosphoric acid respectively, as mobile phase A; other conditions are the same as in Example 1, and take the sample solution described in Example 1 Inject into a liquid chromatography system for analysis, record the chromatogram, and the test results are shown in Table 6.
[0106] Table 6 Comparison of different mobile phase pH examples
[0107]
[0108]
[0109]The experimental results show that when the pH of mobile phase A is 6.0, 5.5, and 6.5, rivaroxaban and its 8 related substances can be separated well, and the content results of various impurities and rivaroxaban are not affected by pH changes Impact proves that the method of the present invention has better method durability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com