Method for identifying base source of trichosanthes kirilowii maxim formula granules
A technology of formula granules and Gualou formula, which is applied in the field of source identification of Gualou formula granules, can solve the problem of being unable to distinguish between Trichosanthes formula granules and double-sided Trichosanthes formula granules, and achieve the effect of simple operation and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This embodiment provides a method for identifying the source of Trichosanthes formula granules, including the following steps:
[0035] (1) Determine the liquid chromatography conditions:
[0036] Chromatographic column: Octadecylsilane bonded silica gel is used as filler, the filler particle size is 1.7μm, column length is 100mm, inner diameter is 2.1mm, column temperature: 35°C;
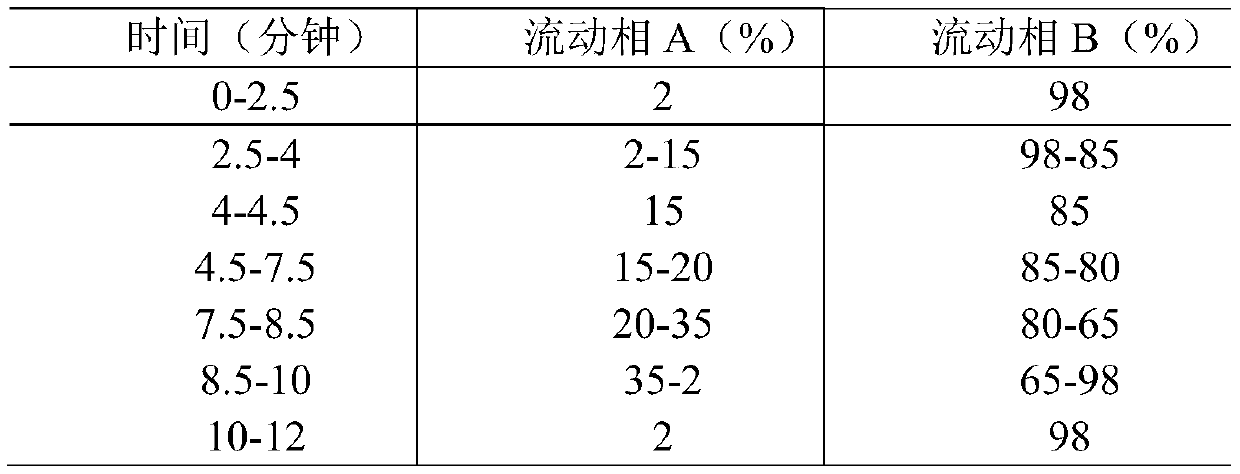
[0037] Mobile phase: mobile phase A is methanol, mobile phase B is 0.1% formic acid aqueous solution, the flow rate is 0.35mL / min, gradient elution is adopted, and the gradient elution procedure is shown in Table 1 below:
[0038] Table 1 Mobile phase gradient elution program
[0039]
[0040] Injection volume: 1 μL; TUV Detector ultraviolet detector.
[0041] (2) Preparation of reference solution and test solution
[0042] Take 1 g of Trichomonas reference medicinal material, add 10 mL of water, ultrasonically extract for 30 minutes, allow to cool to room temperature, shake well, filt...
experiment example 1
[0068] Experimental Example 1 Precision Inspection Test
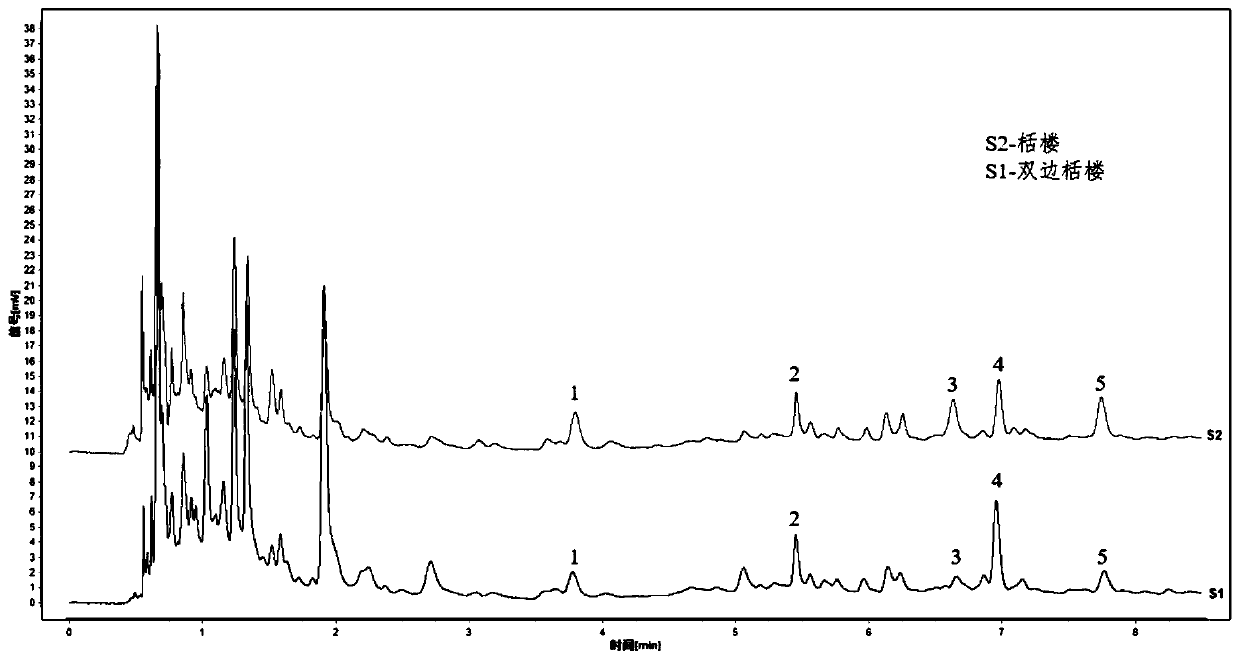
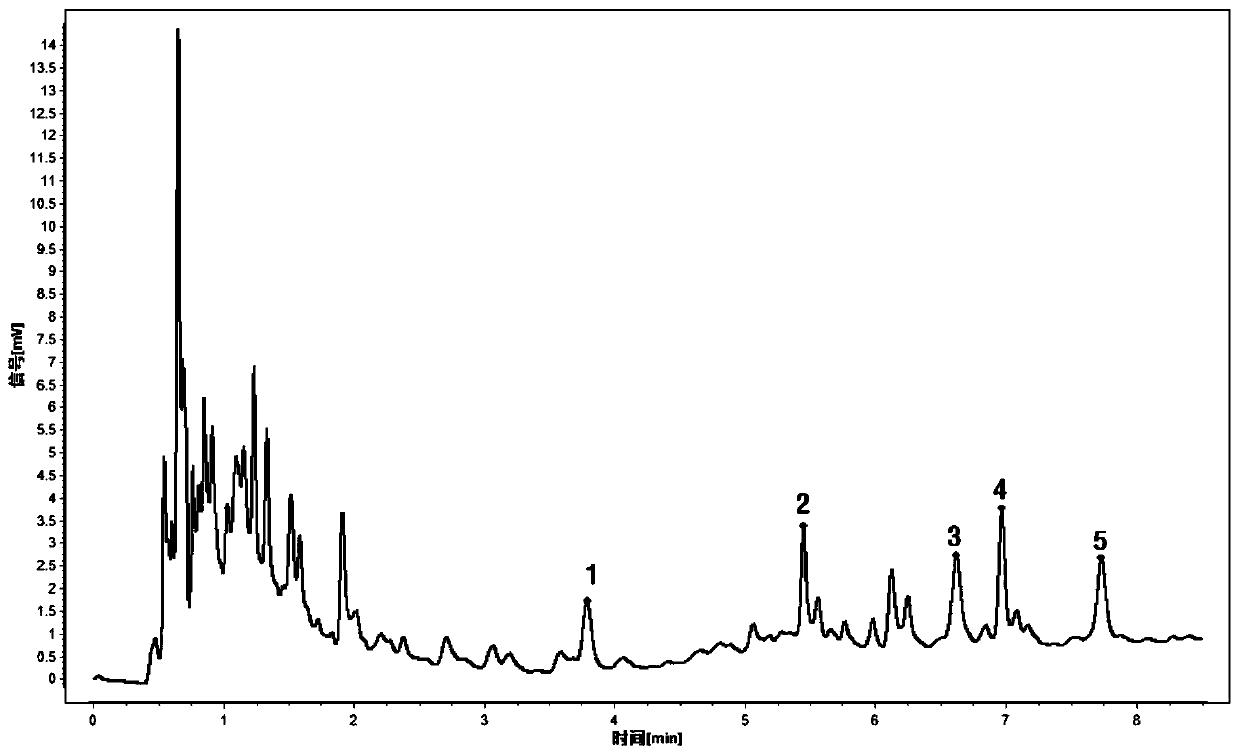
[0069] Get Trichosanthes formula granules (batch number: DF180209-062550-18), make the test solution according to the method of Example 1 step (2), continuously inject 6 times, each 1 μ L, according to Example 1 step (1) The chromatographic conditions are detected, the chromatogram is recorded, the common characteristic peaks are found, and the characteristic spectrum is obtained. Taking peak No. 5 as a reference peak, calculate its relative peak area and relative retention time, and calculate the RSD value. The results are shown in Tables 5 and 6 below.
[0070] Table 5 retention time and relative retention time table
[0071]
[0072] Table 6 Peak area and relative peak area table
[0073]
[0074] From the data in the above table, it can be seen that the relative retention time RSD of each characteristic peak is in the range of 0.03% to 1.25%, and the relative peak area RSD is in the range of 0.65% to 2.39%,...
experiment example 2
[0075] Experimental example 2 Stability investigation test
[0076] Take Trichosanthes formula granules (batch number: DF180209-062550-18), prepare the test solution according to the method of step (2) of Example 1, draw 1 μL at 0, 2, 4, 6, 8, 10, and 12 hours respectively, Inject and detect by the detection condition of embodiment 1 step (1), record chromatogram, take No. 5 peak as reference peak, calculate its relative peak area and relative retention time, and calculate RSD. The results are shown in Tables 7 and 8 below.
[0077] Table 7 retention time and relative retention schedule
[0078]
[0079] Table 8 Peak area and relative peak area table
[0080]
[0081] As can be seen from the data in the above table, the relative retention time RSD of each characteristic peak is in the range of 0.00% to 0.76%, and the relative peak area ratio RSD is in the range of 1.02% to 2.06%, indicating that the chemical composition of the test solution is within 12 hours. Good st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com