Condensed-ring aryl compound, organic electronic device, and application thereof
A technology of fused-ring aromatic groups and compounds, which is applied in the field of optoelectronic materials and can solve the problems of short lifespan of P-doped materials
- Summary
- Abstract
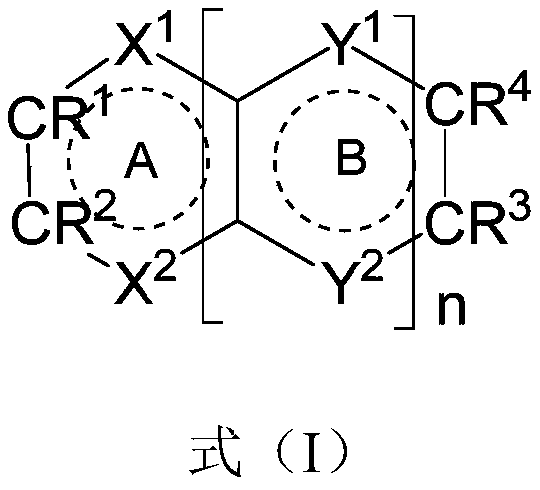
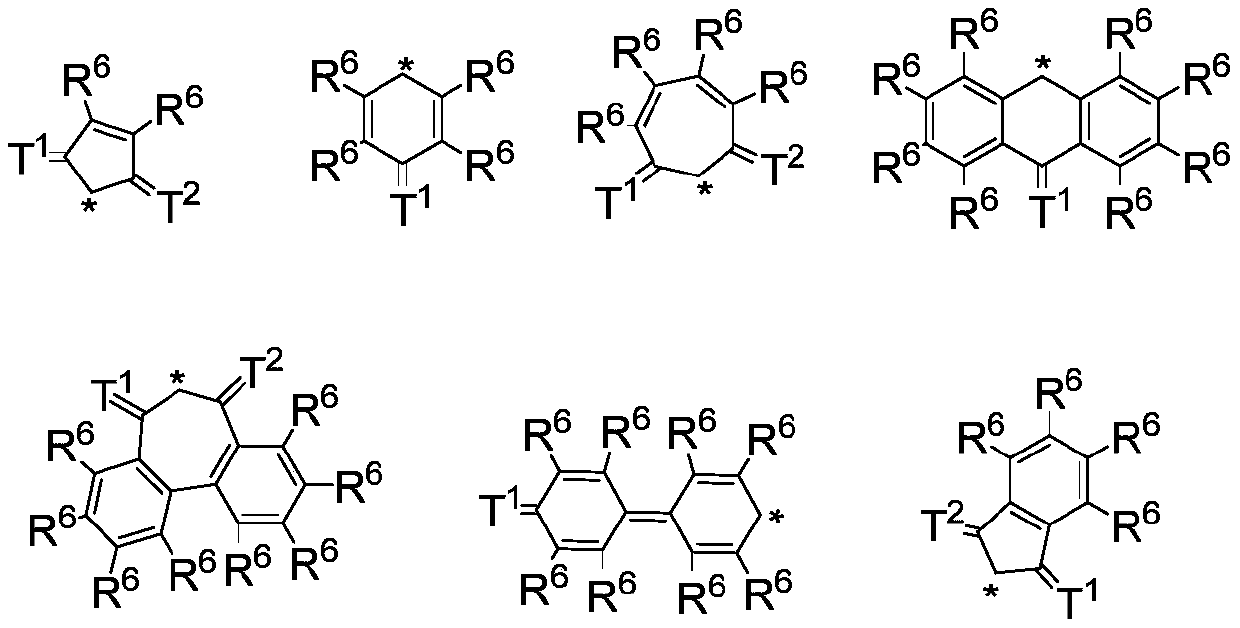
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
[0050] Synthesis of intermediate 1-P-1: In a 250 ml three-necked flask, 1-bromo-2,3,5,6-tetrafluoro-4-nitrobenzene (27.3 g, 1 equivalent, 0.1 mol), 2,3-diaminomaledicyanide (21.60 grams, 2 equivalents, 0.2mol), sodium tert-butoxide (4 equivalents, 0.4mol), ethanol (80 milliliters), reflux, stir 12 hours, react After completion, cool down to room temperature, add 10 ml of ice water to quench, extract with dichloromethane, rotate the concentrate, recrystallize from ethanol, beat with acetonitrile for 3 times to obtain intermediate 1-P-1 (16.2 g, yield 40%) .
[0051] Intermediate 2-P-1: Add intermediate 1-P-1 (4.40 g, 1 equivalent, 0.01 mol), 4-bromo-2,3 , 5,6-tetrafluoropyridine (4.58 grams, 2 equivalents), tetrahydrofuran (THF, 20 milliliters), under the condition of -78°C, slowly add n-butyllithium (2 equivalents), after the addition, it was raised to room temperature and stirred for 4 Hours, after the reaction was completed, 10 ml of water was added dropwise to...
Embodiment 2
[0057]
[0058] Synthesis of P-2: same as the synthesis of intermediate 1-P-1, the difference lies in the substitution of 1,2,4,5-tetrafluoro-3,6-dinitrobenzene (24 g, 1 equivalent, 0.1 mol) 1-bromo-2,3,5,6-tetrafluoro-4-nitrobenzene to obtain compound P-2 (19.0 g, yield 51%).
[0059] Elemental Analysis: C 14 N 10 f 4 Theoretical: C, 45.18; N, 37.63; Found: C, 45.21; N, 37.60; HRMS (ESI) m / z(M): Theoretical: 372.0104; Found: 372.0109.
Embodiment 3
[0061]
[0062] The synthesis of P-3: the same as the synthesis of intermediate 1-P-1, the difference is that 1,2,4,5-tetrafluoro-3,6-dicyanobenzene (20 g, 1 equivalent, 0.1 mol) substituted the compound 1-bromo-2,3,5,6-tetrafluoro-4-nitrobenzene to obtain compound P-3 (14.6 g, yield 44%).
[0063] Elemental Analysis: C 16 N 10 Theoretical: C, 57.84; N, 42.16; Found: C, 57.87; N, 42.13; HRMS (ESI) m / z(M): Theoretical: 332.0307; Found: 332.0312.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com