Preparation method and application of lead-free A4MnBi2X12 perovskite material
A perovskite material, a4mnbi2x12 technology, is used in the preparation and application of new materials, which can solve the problems of difficult to guarantee stability, low luminous quantum efficiency, poor stability, etc., and achieve abundant reserves, environmental friendliness, and good thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] A kind of lead-free A described in the present invention 4 MnB 2x 12 The preparation method of perovskite material, the main steps are:
[0029] Mix halide metal salt, bismuth oxide or its corresponding bismuth salt (bismuth halide, bismuth acetate, bismuth nitrate, etc.), manganese halide / manganese monoxide / manganese acetate, and hydrohalic acid (HX), under high temperature The raw materials are dissolved by keeping warm in a polytetrafluoroethylene hydrothermal kettle, and then slowly lowered to room temperature, the single crystal is precipitated from the hydrohalic acid, filtered and washed to obtain the product A 4 MnB 2 x 12 .
[0030] Among them, the AX, Bi 2 o 3 、MnX 2 In A is Li, Na, K, Rb, Cs, and CH 3 NH 3 + CH 4 N 2 + , C 8 h 11 N + One or more mixtures of inorganic or organic cations, X is one or more mixtures of Cl, Br, I.
[0031] The synthesis method of the invention is simple and reproducible, and the obtained single crystal can be use...
Embodiment 1
[0033] A lead-free A 4 MnB 2 x 12 Perovskite materials (Cs 4 MnB 2 Cl 12 single crystal) preparation method, the main steps are:
[0034] Step 1, 672mg CsCl, 464mg Bi 2 o 3 , 125 mg MnCl 2 Add to 5mL hydrochloric acid, stir for 10 minutes to obtain the precursor;
[0035] Step 2: Transfer the precursor obtained in Step 1 to a polytetrafluoroethylene reactor and seal it; transfer the sealed reactor to an oven at 180°C and keep it warm for 30 minutes. Then slowly lower to room temperature, filter and remove the mother liquor to obtain a single crystal, wash the single crystal with a small amount of ethanol or concentrated hydrochloric acid, and then dry it in an oven at 70°C to obtain the target product Cs 4 MnB 2 Cl 12 single crystal.
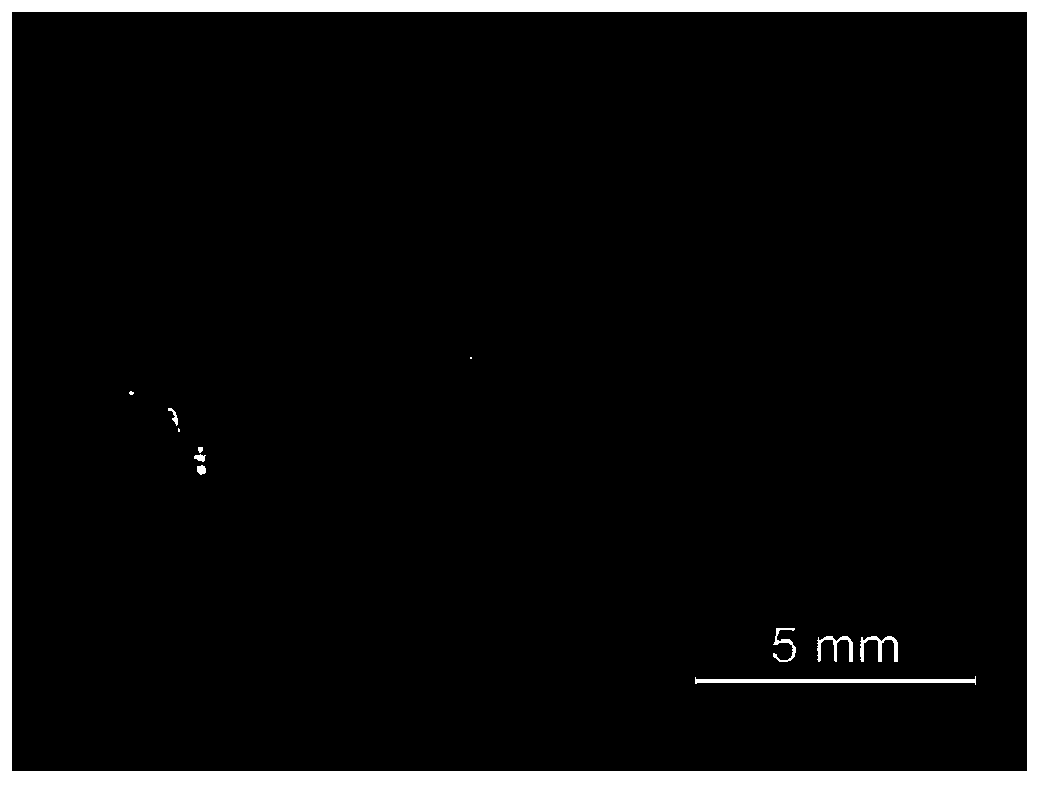
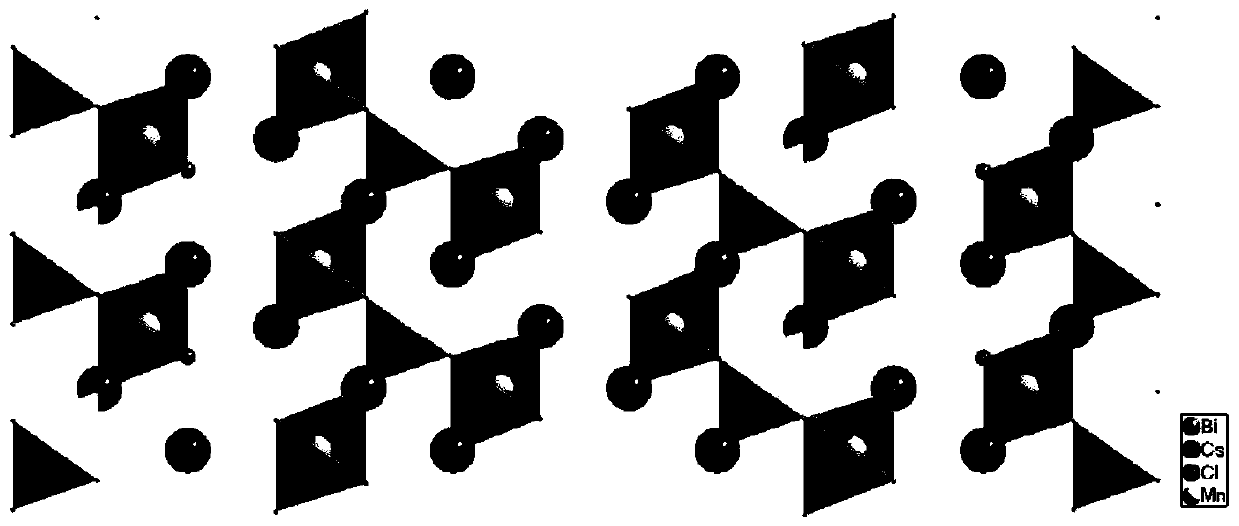
[0036] like figure 1 As shown, the size of the obtained single crystal is about 4mm, and under the excitation of 365nm ultraviolet light, the single crystal emits very bright orange light. The crystal structure of the material was ...
Embodiment 2
[0039] A lead-free A 4 MnB 2 x 12 Perovskite materials (Cs 4 MnB 2 Cl 12 powder), the main steps are:
[0040] Step 1, 672mg CsCl, 464mg Bi 2 o 3 , 125 mg MnCl 2 Add to 5mL of hydrochloric acid, stir for 10 minutes to obtain powder precipitation;
[0041] In step 2, the precipitate obtained in step 1 is centrifuged or filtered, and dried to obtain Cs 4 MnB 2 Cl 12 powder.
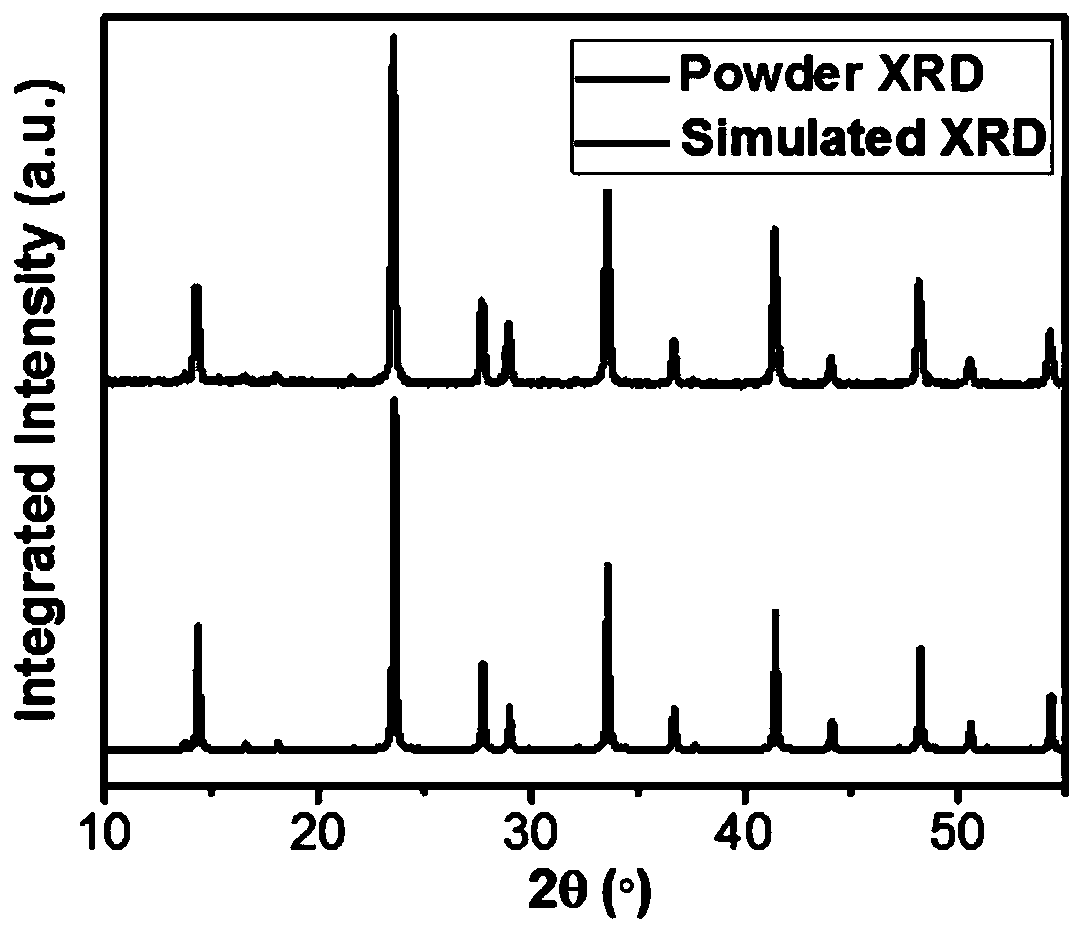
[0042] Cs 4 MnB 2 Cl 12 It can be directly synthesized in hydrochloric acid at room temperature, such as Figure 5a As shown, the XRD of the powder is consistent with the single crystal XRD obtained in Example 1, indicating that the Cs directly synthesized by the normal temperature method 4 MnB 2 Cl 12 The powder phase is consistent with single crystal. Figure 5b It is a photo of the powder under ultraviolet light, and the luminescent color is orange.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Luminous life | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com