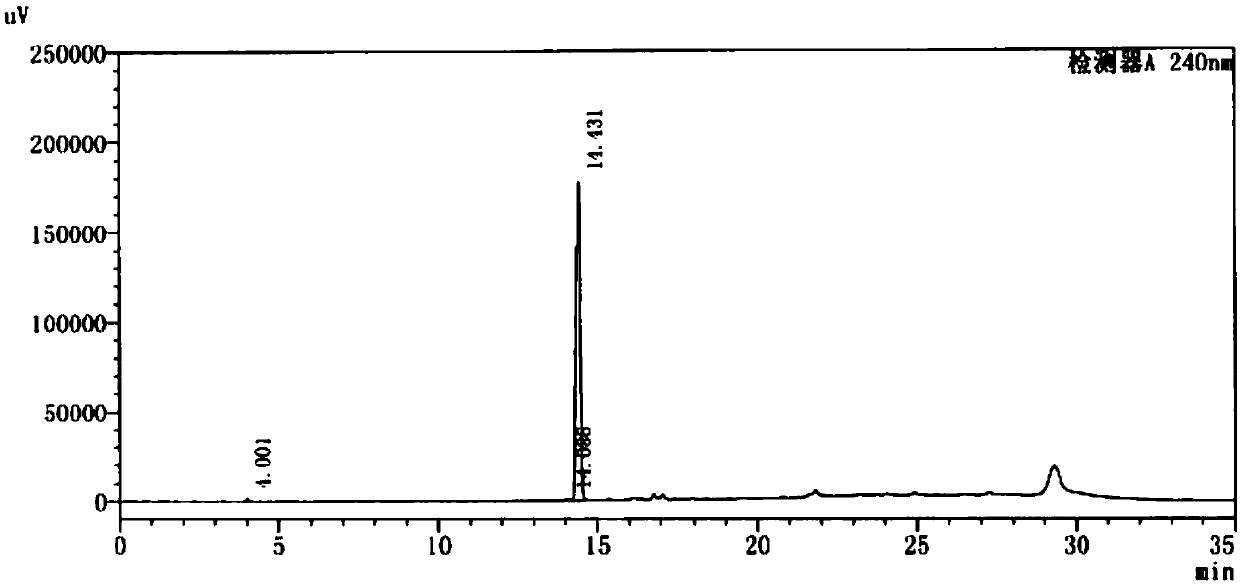
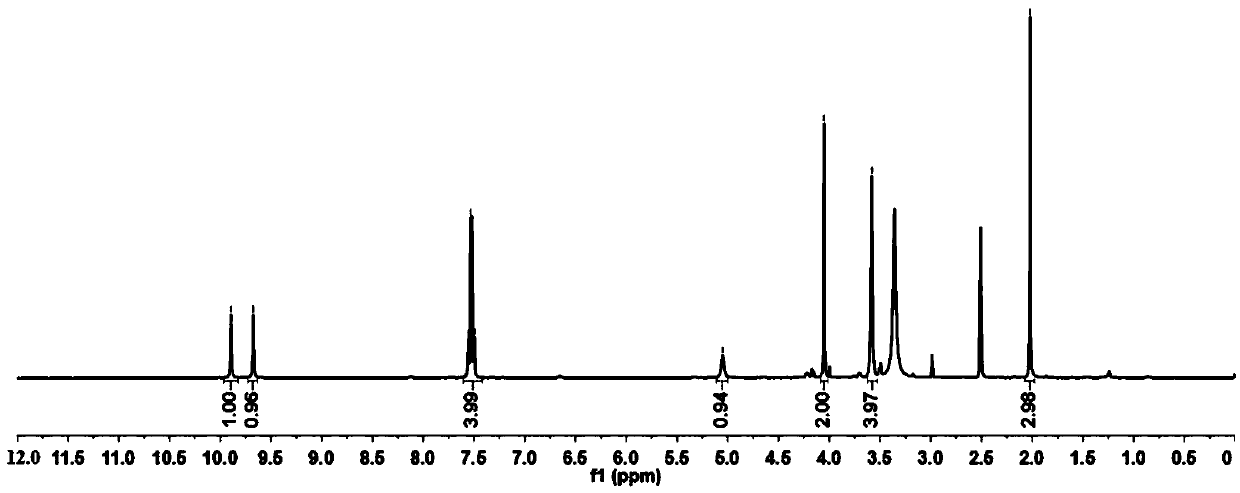
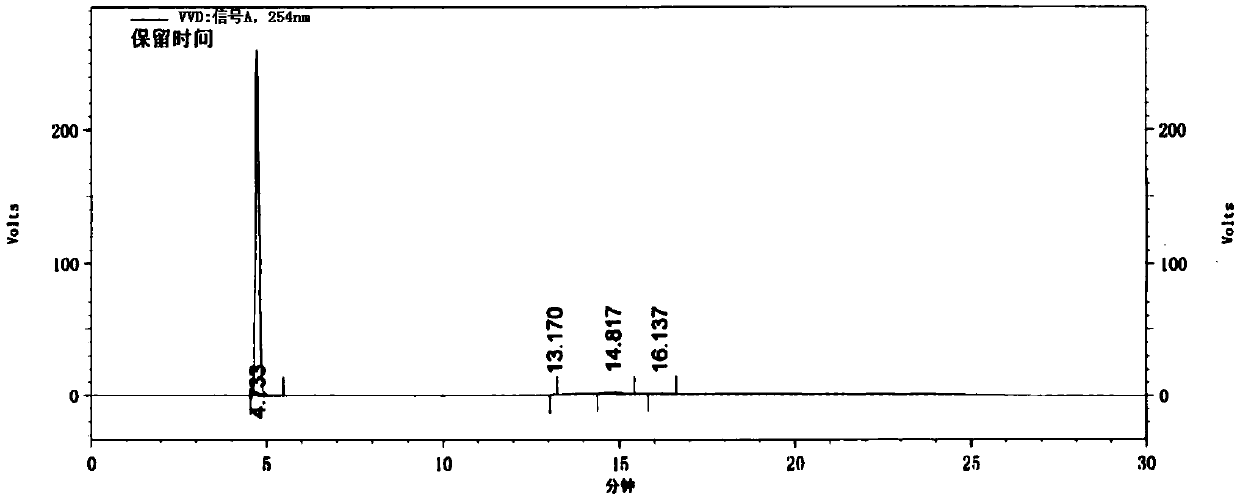
Method for preparing 4-(4-aminophenyl)morpholin-3-one
A technology of aminophenyl and morpholine, applied in the preparation of organic compounds, carboxylic acid amide preparation, chemical instruments and methods, etc., can solve the problems of high activity of nitrohalogenated benzene, high price of bromoacetyl bromide, strong corrosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1: the preparation of p-acetamidoaniline (Ⅲ1)
[0092] Into a 500 ml four-necked flask connected with a stirrer, a thermometer, and a condenser tube, add 360 g of tetrahydrofuran, 69.0 g (0.5 mole) of p-nitroaniline, and 61.2 g (0.6 mole) of acetic anhydride, heat at 60-65 ° C Stir the reaction for 4 hours, detect that the reaction of p-nitroaniline is complete, cool to 20-25 ° C, transfer the resulting reaction liquid to a 1-liter autoclave, wash the four-necked flask with 20.0 grams of water, and transfer the washing liquid to the autoclave. Add 0.8 gram of 5% palladium carbon catalyst (water content 50%), after nitrogen replacement 3 times, stir reaction at internal temperature 40-45 ℃, hydrogen pressure 2-4 atmospheric pressure for 5 hours, cool to room temperature, filter recovery catalyst, use Wash the filter cake with 20 grams of methanol, recover tetrahydrofuran, methanol and aqueous acetic acid from the filtrate, and wash the residue with methyl tert...
Embodiment 2
[0093] Embodiment 2: the preparation of p-acetamidoaniline (Ⅲ1)
[0094] To a 500 ml four-neck flask connected with a stirrer, a thermometer and a condenser tube, add 400 grams of toluene, 69.0 grams (0.5 moles) of p-nitroaniline, and 61.2 grams (0.6 moles) of acetic anhydride, heat at 65-70 ° C Stir the reaction for 5 hours, cool to 20-25°C, transfer the resulting reaction liquid to a 1-liter autoclave, wash the four-necked flask with 20.0 grams of water, transfer the washing liquid to the autoclave, and add 8.0 grams of 50% Raney nickel Catalyst (water content 50%), after nitrogen replacement 3 times, at internal temperature 45-50 ℃, hydrogen pressure 2-4 atmospheric pressure under stirring reaction 6 hours, cool to room temperature, filter recovery catalyst, wash filter cake with 20 grams of methanol, Toluene, methanol, and aqueous acetic acid were recovered from the filtrate, and the residue was washed with methyl tert-butyl ether to obtain 70.6 g of p-acetamidoaniline (Ⅲ1...
Embodiment 3
[0095] Embodiment 3: the preparation of p-acetamidoaniline (Ⅲ1) (separation of p-nitroacetamidobenzene method)
[0096] In the 500 milliliter four-necked flask that is connected with stirrer, thermometer, condenser tube, add 360 grams of dichloromethane, 69.0 grams (0.5 moles) of p-nitroaniline, 61.2 grams (0.6 moles) of acetic anhydride, stir reaction at room temperature for 4 hours , filtered, and the filter cake was washed with 20 grams of dichloromethane to obtain 86.6 grams of p-nitroacetamidobenzene (II), with a yield of 96.1%. The liquid phase purity is 99.5%.
[0097] In the autoclave, successively add 450g tetrahydrofuran, 86.6 grams of p-nitroacetamidobenzene (II) and 8.0 grams of 50% Raney nickel catalyst (water content 50%), after nitrogen replacement 3 times, at an internal temperature of 45-50 ° C, Stir the reaction under 2-4 atmospheres of hydrogen pressure for 8 hours, cool to room temperature, filter and recover the catalyst, wash the filter cake with 20 gram...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com