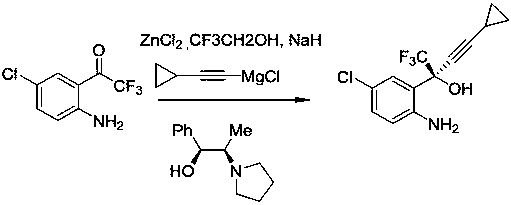
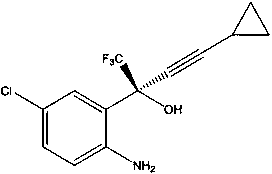
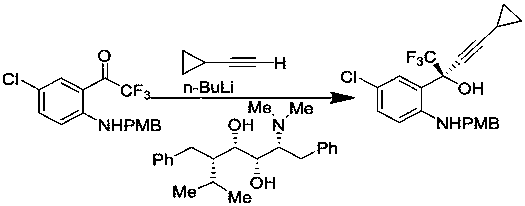
Method for asymmetric synthesis of anti-Aids drug, namely efavirenz key intermediate
An efavirenz and anti-AIDS technology, which is applied in the field of synthesis of the key propargyl alcohol intermediate of efavirenz, can solve the problems of complex operation, expensive ligands, danger, etc., achieve mild process conditions, increase ee value, The effect of short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Under the protection of nitrogen, add 5mL of toluene, 0.0245g of barium fluoride, 0.045g of organic ligand quinidine, and 0.149mL of cyclopropyne into the round-bottomed flask respectively. Stir evenly at a temperature of 25°C, and slowly add dimethyl zinc dropwise. Solution 2.1mL, after the dropwise addition, stir at constant temperature for 3 hours, add tetraisopropyl titanate 0.41mL, continue stirring for 2 hours, then cool down to -35°C, add 5-chloro-2-aminotrifluorobenzophenone in one go 0.157 g, which was stirred at -35°C for 72 hours. TLC detects that the reaction is complete, add 20 mL of toluene to the reaction solution, then slowly add it to 20 mL of dilute hydrochloric acid to quench, then add 2 g of activated carbon and stir for 0.5 hours, then filter, separate the organic phase and the aqueous phase, and wash the organic phase with 100 mL of saturated saline , dried over anhydrous sodium sulfate, and concentrated under reduced pressure at 40°C. After purifi...
Embodiment 2
[0035] Under the protection of nitrogen, add 5mL of toluene, 0.025g of barium fluoride, 0.045g of organic ligand quinidine, and 0.16mL of cyclopropyne into the round-bottomed flask respectively. Stir at a temperature of 25°C and add dimethyl zinc slowly. Solution 2.2mL, after the dropwise addition, stir at constant temperature for 3 hours, add tetraisopropyl titanate 0.42mL, continue stirring for 2 hours, then cool down to -30°C, add 5-chloro-2-aminotrifluorobenzophenone in one go 0.161 g, kept at -30°C and stirred for 72 hours. TLC detects that the reaction is complete, add 20 mL of toluene to the reaction solution, then slowly add 20 mL of dilute hydrochloric acid to quench it, then add 2.5 g of activated carbon and stir for 0.5 hours, then filter, separate the organic phase and the aqueous phase, and use 100 mL of saturated saline for the organic phase Wash, dry over anhydrous sodium sulfate, and distill under reduced pressure at 43°C to obtain 0.163 g of white powder after...
Embodiment 3
[0037] Under the protection of nitrogen, add 6mL of toluene, 0.027g of barium fluoride, 0.046g of organic ligand quinidine, and 0.18mL of cyclopropyne into the round-bottomed flask, stir well at 24°C, and slowly add dimethyl zinc dropwise. Solution 2.4mL, after the dropwise addition, stir at constant temperature for 3.5 hours, add tetraisopropyl titanate 0.43mL, continue stirring for 2 hours, then cool down to -30°C, add 0.17g of 5-chloro-2-aminotrifluorobenzophenone at one time, Stirring was maintained at -30°C for 48 hours. TLC detects that the reaction is complete, add 20 mL of toluene to the reaction solution, then slowly add 20 mL of dilute hydrochloric acid to quench it, add 2.3 g of activated carbon and stir for 0.5 hours, then filter, separate the organic phase and the aqueous phase, and use 100 mL of saturated saline for the organic phase Washed, dried over anhydrous sodium sulfate, and distilled under reduced pressure at 40°C to obtain 0.166g of white powder after pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com