Waterborne polyurethane dispersion with quadrupolar hydrogen bond and preparation method of waterborne polyurethane dispersion
A technology of water-based polyurethane and quadruple hydrogen bond, which is applied in the field of water-based polyurethane dispersion and its preparation, can solve the problems of environmental protection and non-sustainable development, and achieve the effect of high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 (preparation of aminouracil monomer)
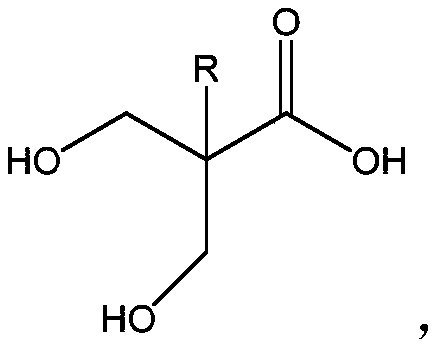
[0046] In the collector-type constant temperature heating magnetic stirrer, install a 250ml single-necked round bottom flask with a condenser tube and a magnetic stirrer, add 12.113g (0.1mol) guanidine carbonate, 19.22g (0.15mol) α-acetyl- γ-butyrolactone 15.18 (0.15mol) and 100ml of absolute ethanol, stirred and heated to reflux for 1 hour, and then the solid-liquid mixed state changed to a brownish-yellow clear and transparent state. After this state lasted for 20 minutes, the clear solution began to appear light yellow. Precipitate, continue to reflux for 5 hours Then stop heating. The supernatant was filtered to obtain a precipitate, that is, a crude product, which was washed with ethanol three times, then stirred with deionized water and adjusted to pH 6-7, filtered and vacuum-dried to obtain a white solid, the final product, aminouracil monomer. 1 H NMR (400MHz, DMSO-d 6 ): δ11.0(1H), 6.4(2H), 4.5(1H), 3.4(2H),...
Embodiment 2
[0047] Embodiment 2 (preparation of water-based polyurethane without quadruple hydrogen bond)
[0048] Add 73g of polybutylene adipate neopentyl glycol ester diol 2000 (PBNA2000) into a 500ml three-necked round-bottomed flask equipped with an electric stirrer, a condenser, and a thermometer, and vacuum dehydrate at 130°C and 0.01MPa for 2 hours. Cool down to 60°C and add 19.05g of isophorone diisocyanate (IPDI), 14g of N,N-dimethylacetamide, 0.02g of stannous octoate catalyst and 1.03g of 1,4-butanediol to adjust the soft and hard segments of the blank sample reagent, heated to 95°C, and reacted for 1.5h. Cool down to 60°C, add 2.412g of 2,2-dimethylolpropionic acid (DMPA), raise the temperature to 85°C, react for 2h, titrate the remaining amount of -NCO with di-n-butylamine until the -NCO content reaches 1.66wt%. Cool down to 60°C, add 150g of acetone to dissolve. The temperature of the system was controlled at 45° C., and 1.818 g of triethylamine, 2.085 g of isophoronediam...
Embodiment 3
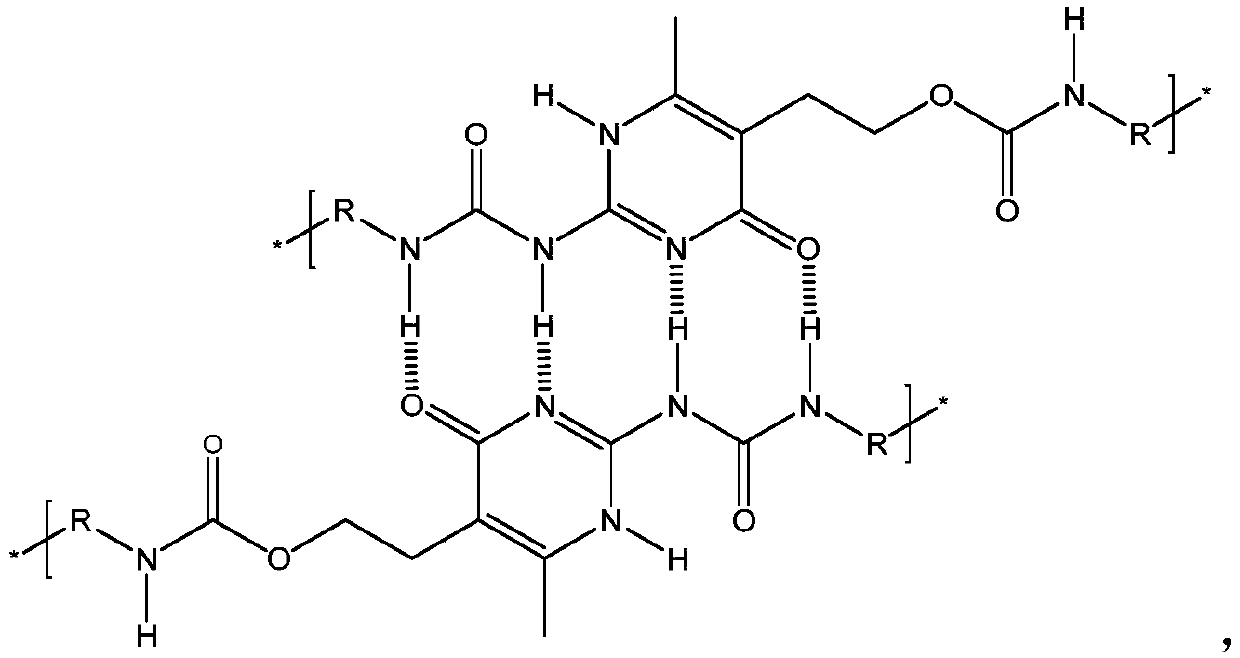
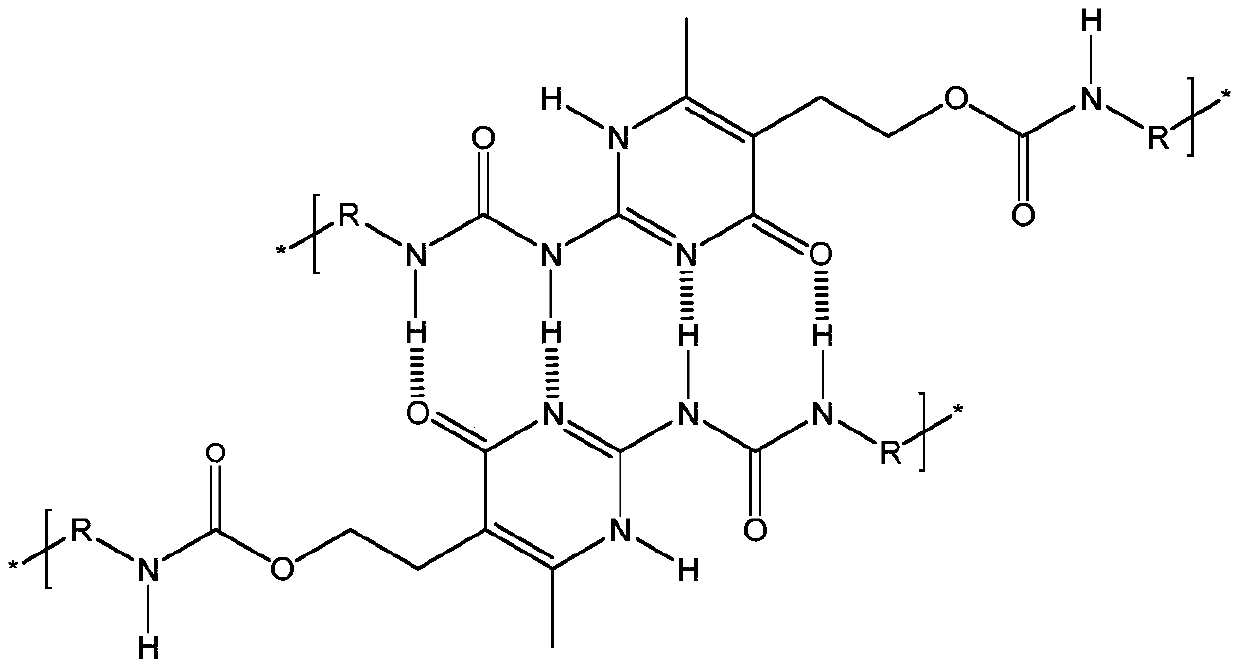
[0049] Embodiment 3 (preparation of waterborne polyurethane containing quadruple hydrogen bond)
[0050] Add 73g polybutylene adipate neopentyl glycol ester diol 2000 (PBNA2000) and 0.676g aminouracil monomer (HMA) in the 500ml three-neck round bottom flask that electric stirrer, condenser, thermometer are housed, in Vacuum dehydration at 130°C and 0.01MPa for 2 hours. When the temperature was lowered to 60°C, 18.64g of isophorone diisocyanate (IPDI), 14g of N,N-dimethylacetamide and 0.02g of stannous octoate catalyst were added, the temperature was raised to 95°C, and the reaction was carried out for 1.5h. Cool down to 60°C, add 2.412g of 2,2-dimethylolpropionic acid (DMPA), heat up to 85°C, react for 2h, titrate the remaining amount of -NCO with di-n-butylamine until the -NCO content reaches 2.13wt% . Cool down to 60°C, add 150g of acetone to dissolve. The temperature of the system was controlled at 45° C., and 1.818 g of triethylamine, 2.843 g of isophoronediamine and 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com