Preparation method and application of tadalafil cis-intermediate
A non-cis-intermediate technology, applied in the field of preparation of tadalafil cis-intermediate, can solve the problems of unfavorable industrial production, cumbersome operation, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
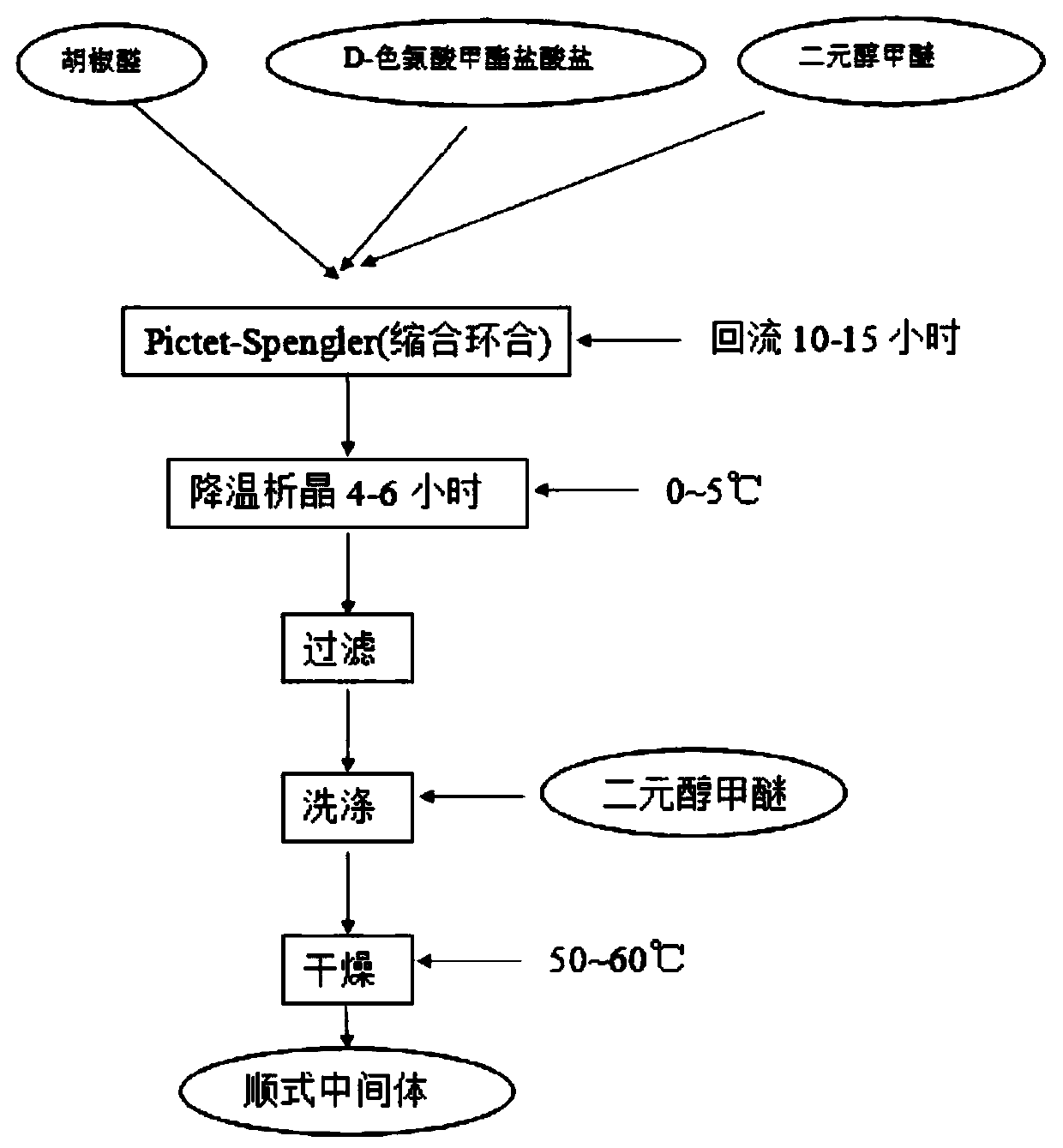
[0031] Such as figure 1 As shown, the preparation method of the tadalafil cis intermediate provided by the embodiments of the present invention comprises the following steps:
[0032] S101: using D-tryptophan methyl ester hydrochloride as a starting material;
[0033] S102: Mixing with piperonal in a glycol ether solution to obtain a cis intermediate.
[0034] The ratio of D-tryptophan methyl ester hydrochloride to piperonal is 1:1.1-1.5, and the ratio of D-tryptophan methyl ester hydrochloride to glycol ether is listed as 1 g / 4 ml.
[0035] The preparation method of the tadalafil cis intermediate provided in the examples of the present invention uses D-tryptophan methyl ester hydrochloride I as the starting material, and mixes it with piperonal II in a glycol ether solution to obtain the cis Intermediate III. The chemical reaction formula is as follows:
[0036]
[0037] Further, as a preferred embodiment of the present invention, the glycol ethers are: ethylene glycol...
Embodiment 7
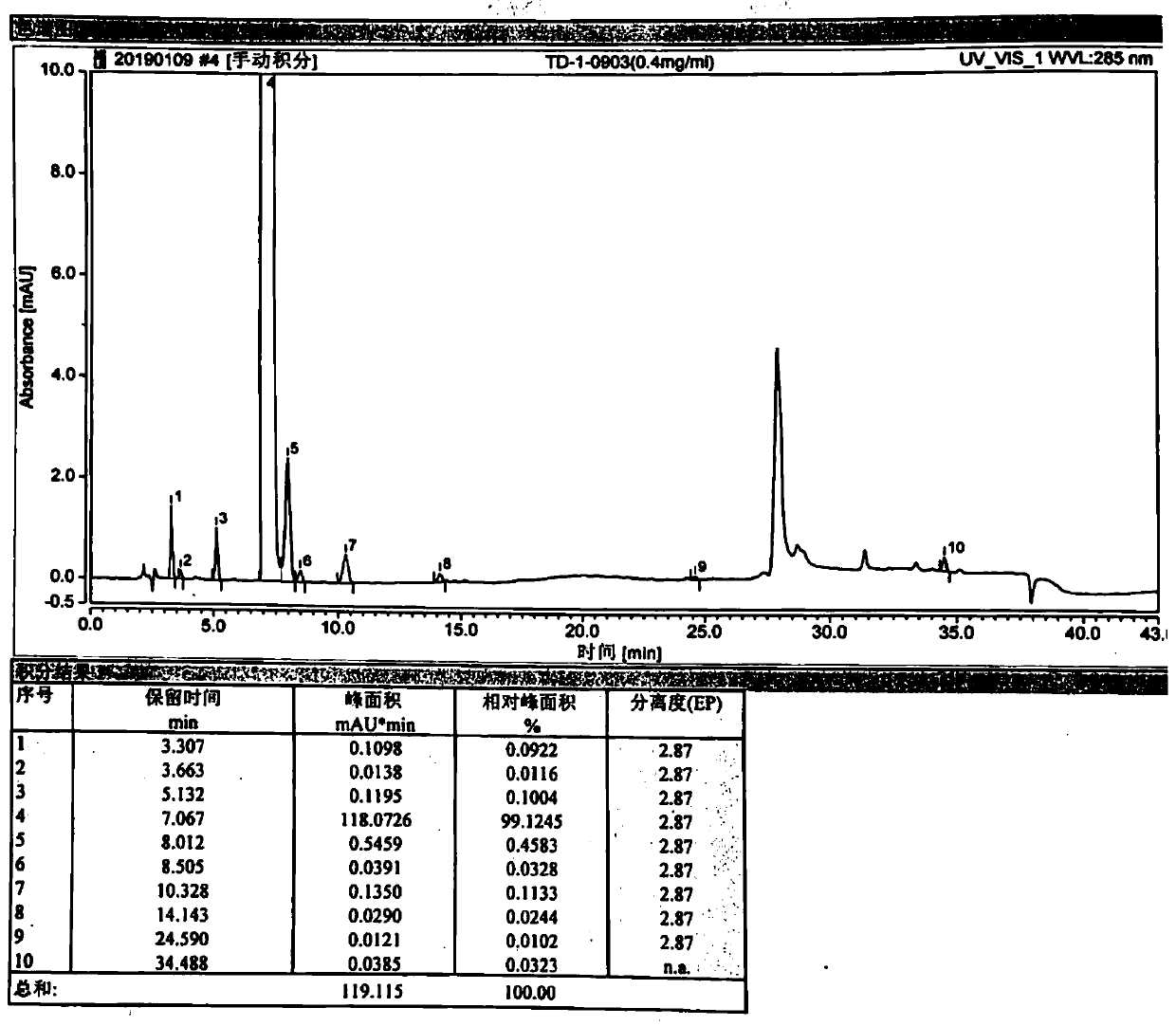
[0054] Example 7 cis intermediate HPLC detection conditions
[0055] (1) Chromatographic column: octaalkylsilane bonded silica gel column
[0056] (2) Mobile phase: 0.1% trifluoroacetic acid was used as mobile phase A, and acetonitrile was used as mobile phase B.
[0057] (3) Detection wavelength: 285nm
[0058] (4) Flow rate: 1.0ml / min
[0059] (5) Column temperature: 40°C
[0060] (6) Gradient elution program
[0061]
[0062]
[0063] (7) Determination method
[0064] According to the "Chinese Pharmacopoeia" 2015 edition four general rules 0512 high performance liquid chromatography test, according to the above chromatographic conditions, accurately measure 10ul of the test solution, inject it into the liquid chromatograph, record the chromatogram, and calculate the relative peak of the main peak according to the peak area normalization method. percentage content.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com