Polyphosphazene covalent triazine polymer and preparation method and application thereof
A technology of covalent triazine and phosphazene compounds, which is applied in the field of polyphosphazene covalent triazine polymers and their preparation, can solve the problems of complex preparation process, limitation of practical application, and damage to the mechanical properties of polymer substrates, and achieve The effect of simple preparation process, great practical application potential and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
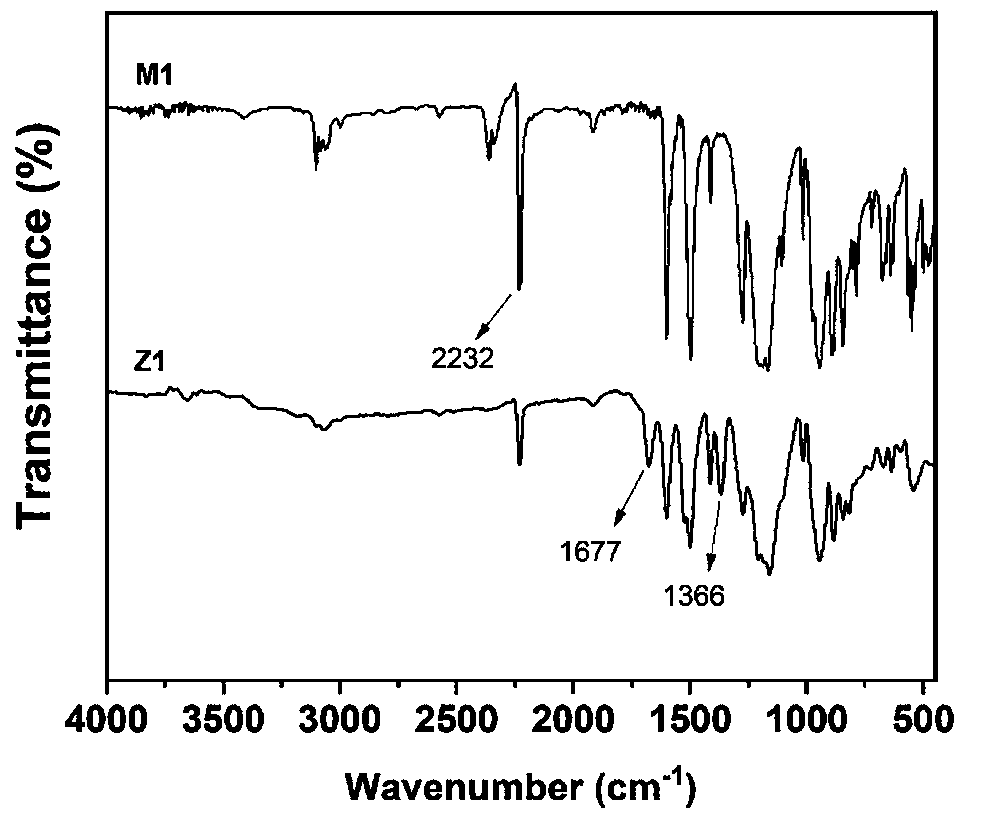
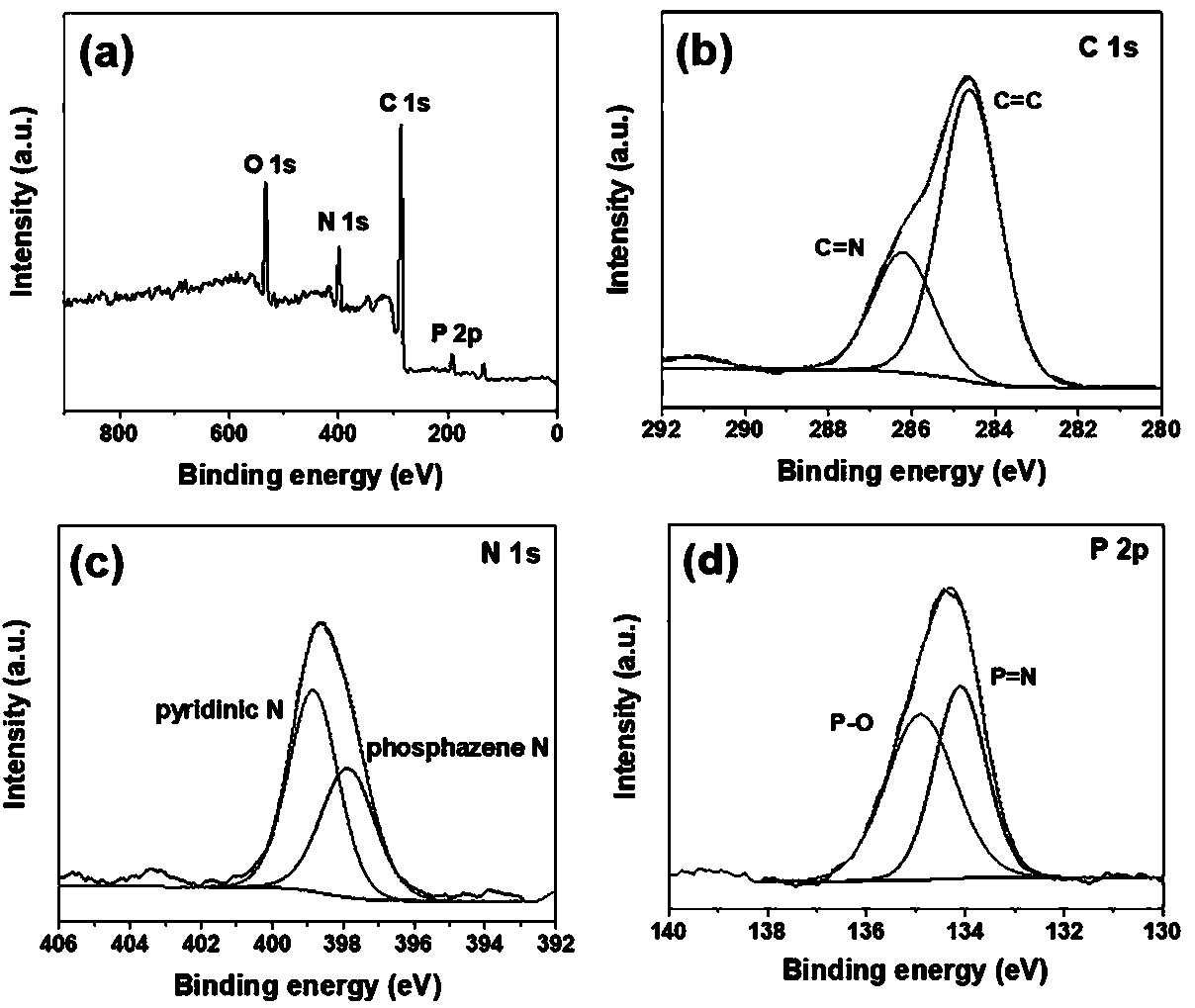
[0041] (1) Under the protection of nitrogen, dissolve 57.44 mmol HCCP, 344.8 mmol 4-hydroxybenzonitrile and 828 mmol potassium carbonate in 800 mL acetone, then react at 55 °C for 10 h, after the reaction, remove the excess by rotary evaporation solvent, and washed several times with a large amount of deionized water, and then suction filtered, and the crude product was recrystallized and dried to obtain the cyano-containing phosphazene compound M1.
[0042] (2) Under nitrogen protection, disperse 18.5 mmol of cyano-containing phosphazene compound M1 in chloroform, control the temperature at 0 °C, and gradually add 120 mmol of trifluoromethanesulfonate to the above system using a constant pressure dropping funnel The acid was dropped within 30 min and kept at 0 °C for 2 h, then at room temperature for 22 h. After the reaction, 1.0 mol / L ammonia solution was added to adjust the pH of the system to be neutral, and stirred for 2 h. Finally, the obtained product was filtered, was...
Embodiment 2
[0048](1) Under the protection of nitrogen, dissolve 57.44 mmol HCCP, 344.8 mmol 4-hydroxybiphenonitrile and 828 mmol triethylamine in 800 mL acetone, then react at 55 °C for 24 h, after the reaction, remove by rotary evaporation Excess solvent, washed several times with a large amount of deionized water, and then suction filtered, recrystallized and dried the crude product to obtain the cyano-containing phosphazene compound M2.
[0049] (2) Under nitrogen protection, disperse 18.5 mmol of cyano-containing phosphazene compound M2 in chloroform, control the temperature at 0 °C, and gradually add 120 mmol of trifluoromethanesulfonate to the above system using a constant pressure dropping funnel The acid was dropped within 30 min and kept at 0 °C for 2 h, then at room temperature for 22 h. After the reaction, 0.5 mol / L ammonia solution was added to adjust the pH of the system to neutral, and stirred for 2 h. Finally, the obtained product was filtered, washed several times with d...
Embodiment 3
[0051] (1) Under the protection of nitrogen, 57.44 mmol HCCP, 172.4 mmol 4-hydroxybenzonitrile, 172.4 mmol phenol and 828 mmol potassium carbonate were dissolved in 800 mL acetone, and then reacted at 55 °C for 48 h. After the reaction, The excess solvent was removed by rotary evaporation, washed several times with a large amount of deionized water, and then suction-filtered, and the crude product was recrystallized and dried to obtain the cyano-containing phosphazene compound M3.
[0052] (2) Under nitrogen protection, disperse 18.5 mmol of cyano-containing phosphazene compound M3 in chloroform, control the temperature at 0 °C, and gradually add 90 mmol of trifluoromethanesulfonate to the above system using a constant pressure dropping funnel The acid was dropped within 30 min and kept at 0 °C for 2 h, then at room temperature for 22 h. After the reaction, 0.5 mol / L ammonia solution was added to adjust the pH of the system to neutral, and stirred for 2 h. Finally, the obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
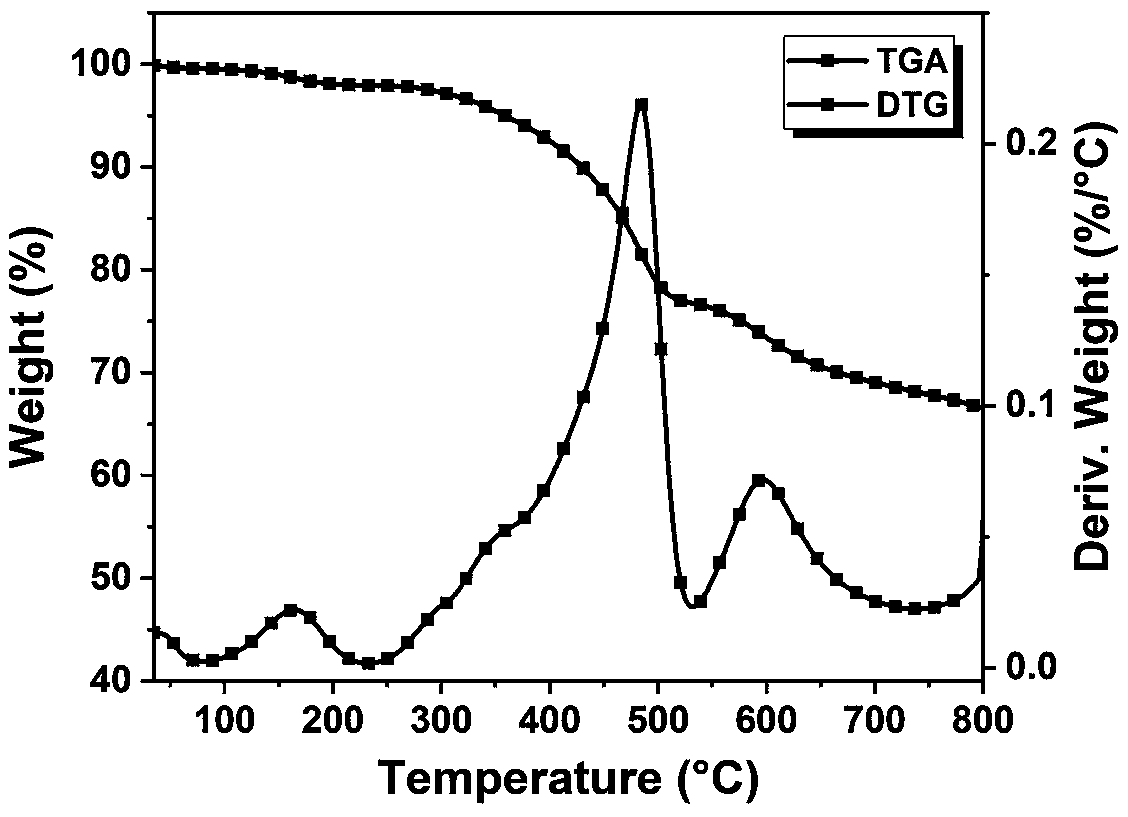
| limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com