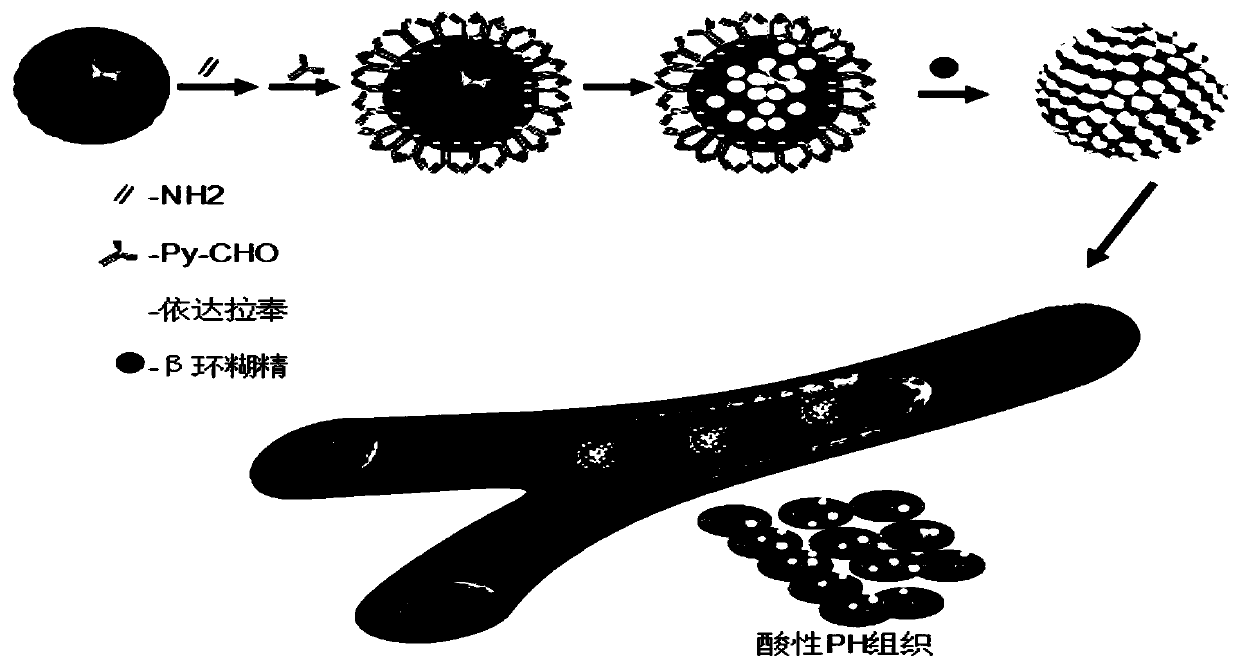
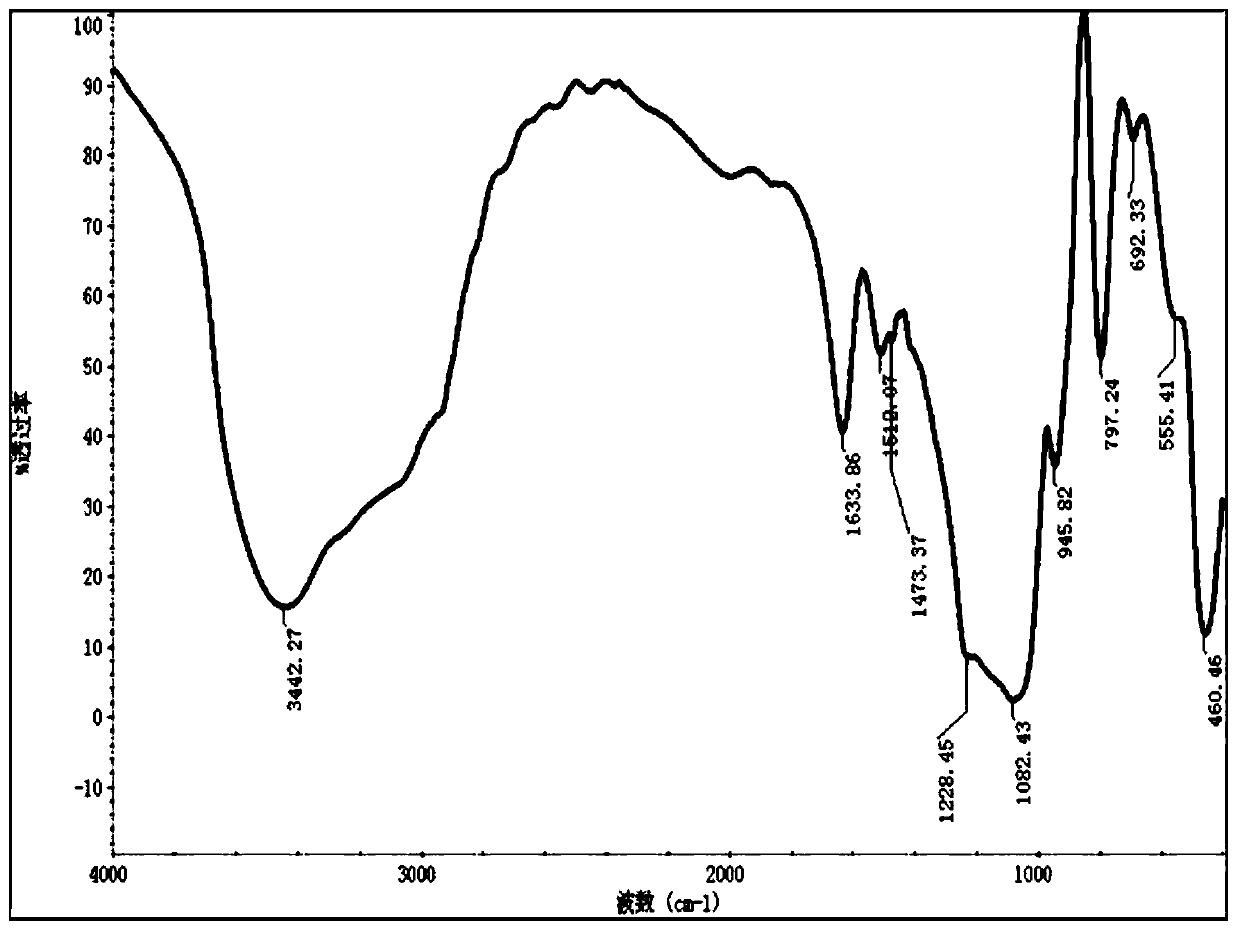
Drug controlled-release mesoporous silicon nanoparticles and preparation method thereof
A technology of silicon nanoparticles and nanoparticles, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., can solve the problems of poor pH response and drug controlled release performance, and achieve good effects , good drug release performance, and good responsiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A preparation method of drug controlled-release mesoporous silicon nanoparticles according to an embodiment of the present invention, the preparation method comprising the following steps:
[0036] (1) Synthesis of mesoporous silicon nanoparticles
[0037] Weigh 0.50g of cetyltrimethylammonium bromide (CTAB) and dissolve it in 240mL of deionized water, add 1.75mL of 2.0M NaOH solution, adjust the temperature to 70°C, keep warm for 30min, and quickly add 2.75mL of Ethyl silicate (TEOS), after 1 min, add 2.50 mL of ethyl acetate, continue to stir for 2 h, until a white precipitate appears, wash with ultrapure water and ethanol (three times) after centrifugation, and vacuum dry overnight at 60 ° C to obtain a white solid Mesoporous silicon nanoparticles;
[0038] (2) Amination modification of mesoporous silicon nanoparticles
[0039] Weigh 100 mg of the prepared mesoporous silicon nanoparticles, add 50 μL of 3-aminopropyltriethoxysilane (APTES), reflux in 10 mL of dry to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com