13-aminoxanthatin derivatives and preparation method and application thereof
A technology of Xanthomonas and derivatives, which is applied in the field of 13-aminoxanthium derivatives and their preparation, can solve problems such as no reports on activity research, and achieve the effect of strong poisoning activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
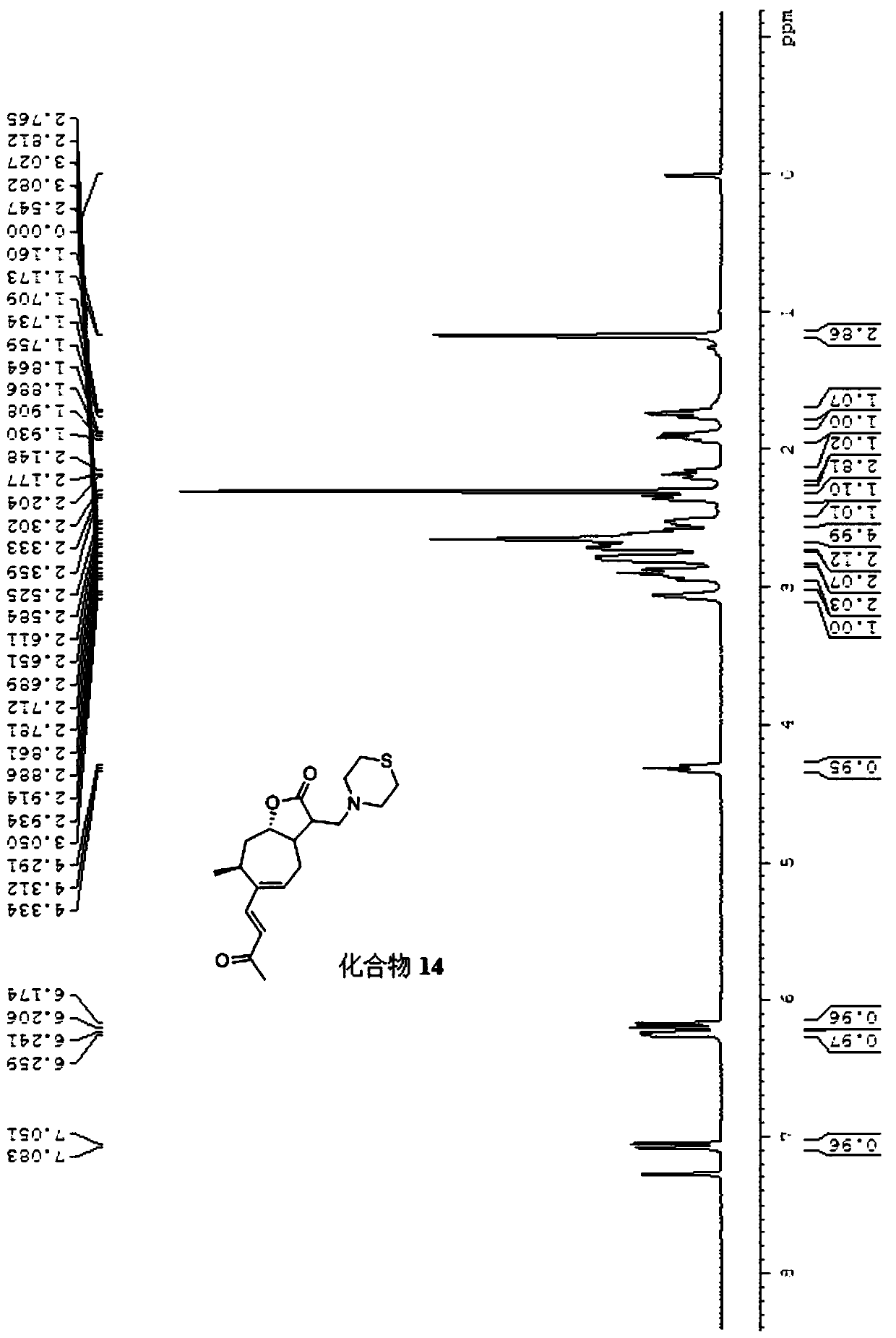
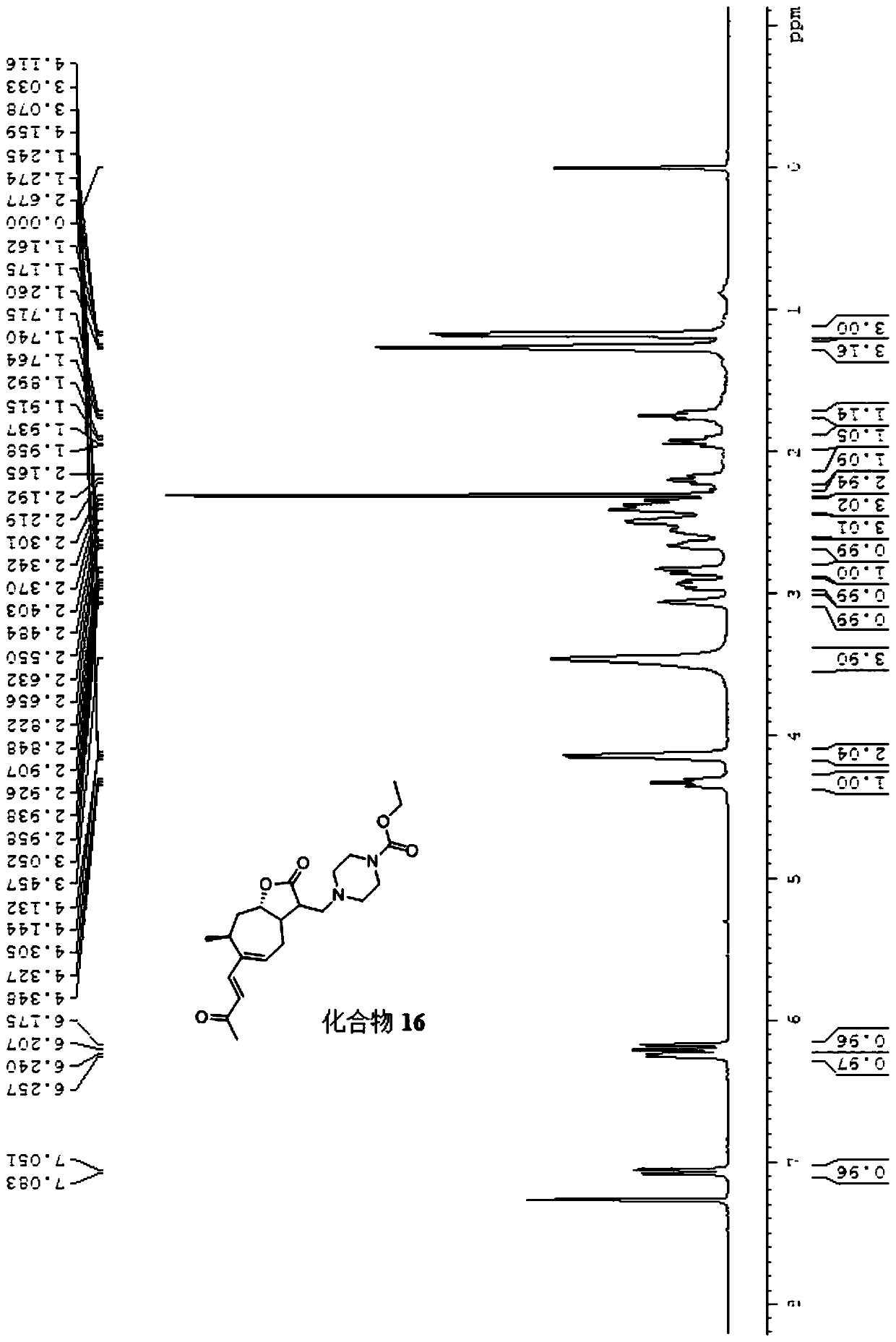
[0020] The following is the synthetic route of compound 1-20:
[0021] Dissolve a certain amount of Xanthin and secondary amine in methanol, then stir and react at room temperature or in an oil bath, and track and detect with TLC. After the reaction is completed, concentrate the reaction solution under reduced pressure, and prepare silica gel thin plate chromatography to obtain the desired target product.
[0022] The numbers of compounds 1-20 correspond to the numbers (1)-(20) of R in the general chemical formula respectively.
[0023]
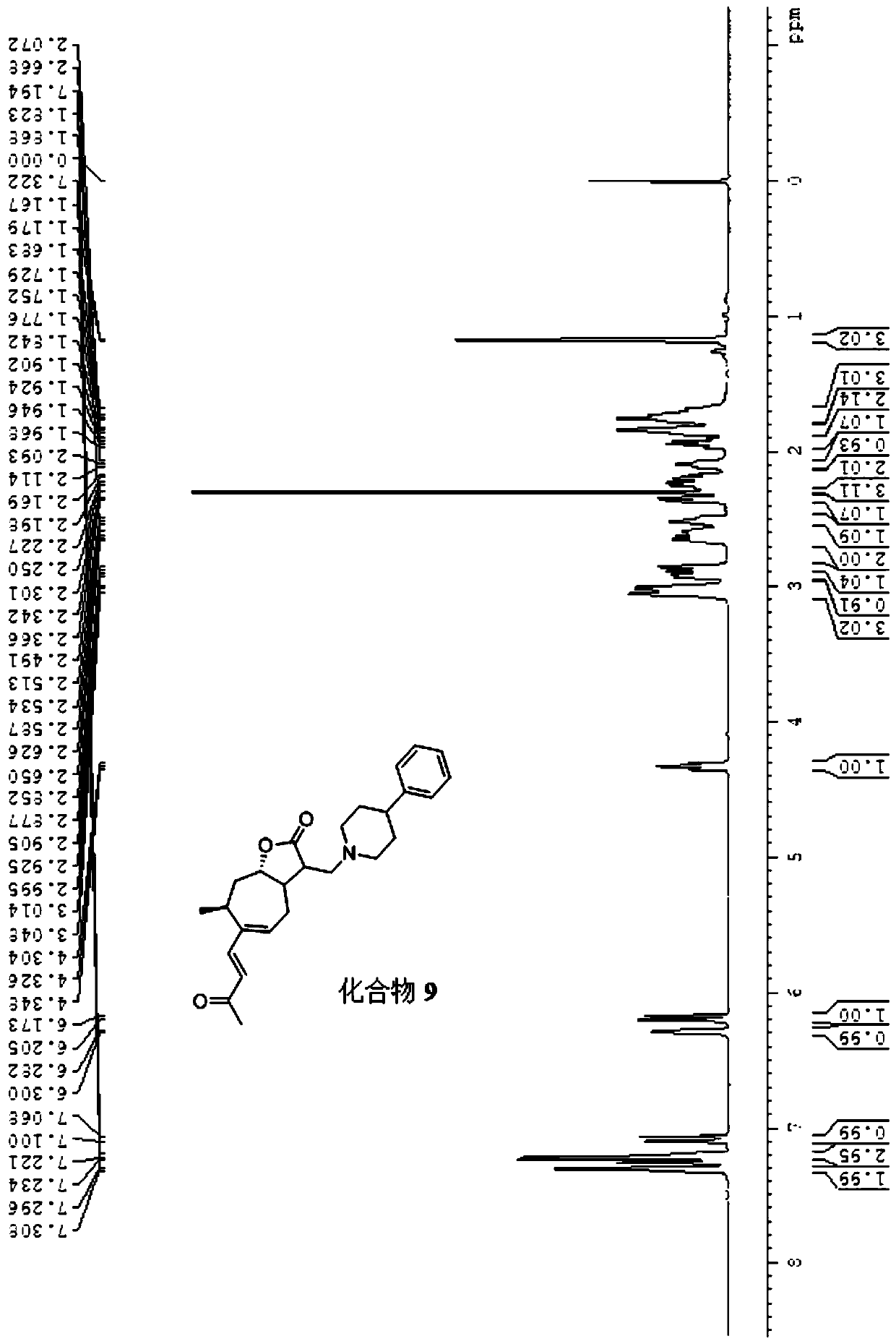
[0024] The physical and chemical properties of compound 1 are as follows:
[0025] 1) Pale yellow liquid;
[0026] 2) The nuclear magnetic resonance spectrum characteristic of this compound:
[0027] With deuterated chloroform as solvent, TMS as internal standard, wherein each peak belongs to: 1 H NMR (500MHz, CDCl 3 )δ: 7.07 (d, J = 16.0Hz, 1H, 2-H), 6.26 (d, J = 9.0Hz, 1H, 5-H), 6.19 (d, J = 16.0Hz, 1H, 3-H) ,4.29-4.34(m,1H,8-H),3....
Embodiment 2
[0148] Insecticidal activity test:
[0149] 1. Insects to be tested: larvae of diamondback moth (Plutella xylostella) at the end of the 2nd instar.
[0150] 2. Samples and reagents: raw material Xanthin, Xanthin derivatives 1-20 prepared in the examples, and positive control drug toosendanin (>98%); solvent is acetone (analytical pure).
[0151] 3. Bioassay method:
[0152] Using the small leaf dish addition method: spread a layer of filter paper on the bottom of a 9cm-diameter petri dish, and add water to moisturize it. Pick 20 larvae of Plutella xylostella xylostella at the end of the second instar with the same size and robustness in each dish. Weigh appropriate amounts of toosendanin, xanthin and compound 1-20 prepared in the examples and add acetone to prepare a drug solution with a concentration of 20 mg / ml. Cut the cabbage leaves into 1×1cm small leaf discs, use a micro-dropper to measure 2 μL of the drug solution to be tested and apply it evenly on the surface of the ...
Embodiment 3
[0159] Antibacterial activity test:
[0160] 1. Pathogens tested: Alternaria solani, Fusarium solani, Botrytis cinerea, Colletotrichum morbiculare, provided by the Plant Pathology Laboratory of Shanxi Agricultural University.
[0161] 2. Samples and reagents:
[0162] The samples are: raw material Xanthin, Xanthin derivatives 1-20 prepared in the examples, and positive control agents difenoconazole (98%) and hymexazol (99%); the solvent is DMSO (chromatographically pure) , the emulsifier is Tween-80 (excellent pure).
[0163] 3. Bioassay method:
[0164] Using the method of inhibiting spore germination:
[0165] (1) Preparation of spore suspension: add sterile water to the cultured plate of sporulated strains, gently rub the surface of the culture medium with an inoculation loop, and then filter out mycelia and culture medium with double-layer gauze. Add appropriate sterile water to the spore suspension and stir. Observe under a 100X microscope, preferably 30-40 spores pe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com