Novel insect kinin analogue and application thereof in aphid prevention and control
A technology of drugs and compounds, applied in the field of agriculture, can solve the problem that the biological activity of kinin analogs is not very prominent, and achieve the effect of obvious aphidicidal activity and good control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
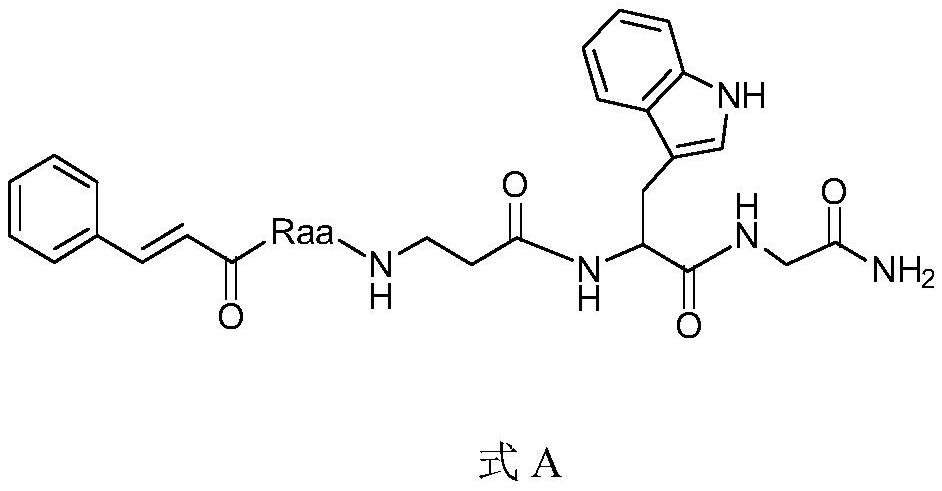
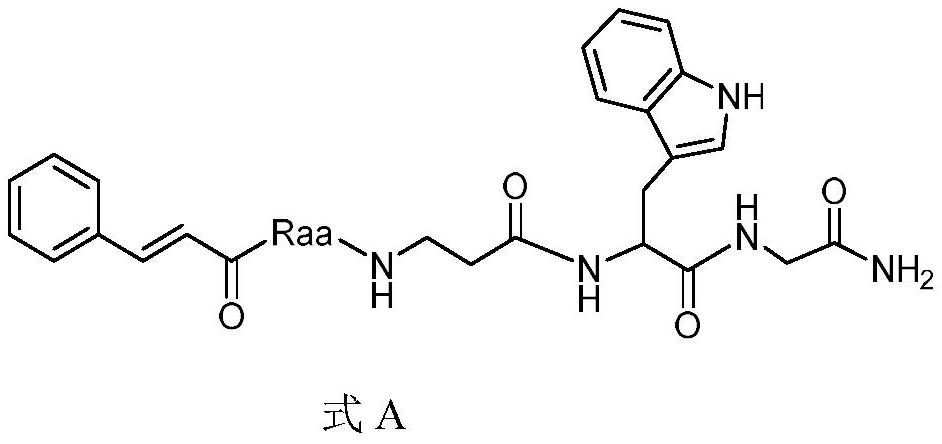
[0019] The preparation of the compound shown in embodiment 1, AI-1 (Raa comes from glutamic acid-5-cyclohexyl ester)
[0020] After checking the air tightness of the peptide synthesizer, take Rink Amide-Am resin (0.3mmol) and place it in 5mL DCM for activation for 2h, then wash it with DMF for 5 times, add 5mL of 20% piperidine in DMF and react for 20min to remove the resin Add Fmoc protecting group; prepare 5mL DMF solution containing Fmoc-Gly-OH (1.2mmol), HBTU (1.2mmol), HOBt (1.2mmol), DIEA (1.2mmol), and react with the resin for 2h at room temperature after activation for 5min Get Fmoc-Gly with Rink Amide-Amresin. Continue to remove the Fmoc group, and insert Fmoc-Trp(Boc)-OH, Fmoc-β-Ala-OH, Fmoc-glutamate-5-cyclohexyl and cinnamic acid sequentially in the same way. Finally, a mixed solution of trifluoroacetic acid: phenol: sulfide anisole: water = 90:5:2.5:2.5 was used to react with the resin for 4 hours to obtain the target product. Filter to remove TFA, add an approp...
Embodiment 27
[0076] Embodiment 27, the biological activity of the compound of the present invention to soybean aphid (Aphis glycines Matsmura)
[0077] The insecticidal activity of the compound of the present invention on aphids is determined by a leaf-dipping method. The target compound was made into a 200 mg / L assay solution. Then use a 1-5ml pipette gun to take 1mL DMSO and add it to a weighing bottle, add 9mL aqueous solution containing 0.1% Triton X-100, mix well to obtain a 200mg / L measurement solution, and then use 0.1% Triton X-100 The aqueous solution of X-100 is diluted step by step, and mixed thoroughly to obtain the desired concentration. Cultivate soybean aphids and soybean leaves that have not been exposed to any pesticides indoors, use a puncher with a diameter of 15mm to punch out leaves of appropriate size, immerse them in the diluted medicinal solution for 15 seconds, and take them out to dry. Then the leaves were put into a bioassay plate, 1.5% agar was added to the bo...
Embodiment 28
[0085] Example 28, the biological activity of some compounds of the present invention on pea aphid (Acyrthosiphon pisum), green peach aphid (Myzuspersicae (Sulzer))
[0086] The insecticidal activity to pea aphid and green peach aphid was also determined by the leaf-dipping method described in Example 2, and the results are shown in Table 3 and Table 4, respectively.
[0087] Insecticidal activity of some compounds in table 3 formula A to pea aphid (Acyrthosiphon pisum)
[0088]
[0089] After activity determination, it was found that this kind of insect kinin compounds also has good activity against pea aphid, and the compounds AI-1 and AI-8 have good activity on the LC of pea aphid. 50 are lower than pymetrozine, indicating that their activity against aphids is better than that of commercial drug pymetrozine.
[0090] The insecticidal activity of table 4 formula part A compound to green peach aphid (Myzus persicae (Sulzer))
[0091]
[0092] After testing the insecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com