Recombinant porcine circovirus type 2 Cap protein with tandem dominant epitope, and application thereof
A porcine circovirus, dominant epitope technology, applied in the direction of application, virus, viral peptide, etc., can solve the problems of inability to completely prevent PCV2 infection or transmission, incomplete inactivation, poor protection rate, etc., and achieve good immune protection, Good reactogenicity, the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1 Construction of recombinant baculovirus transfer vector
[0055] (1) Gene sequence design and synthesis of recombinant proteins
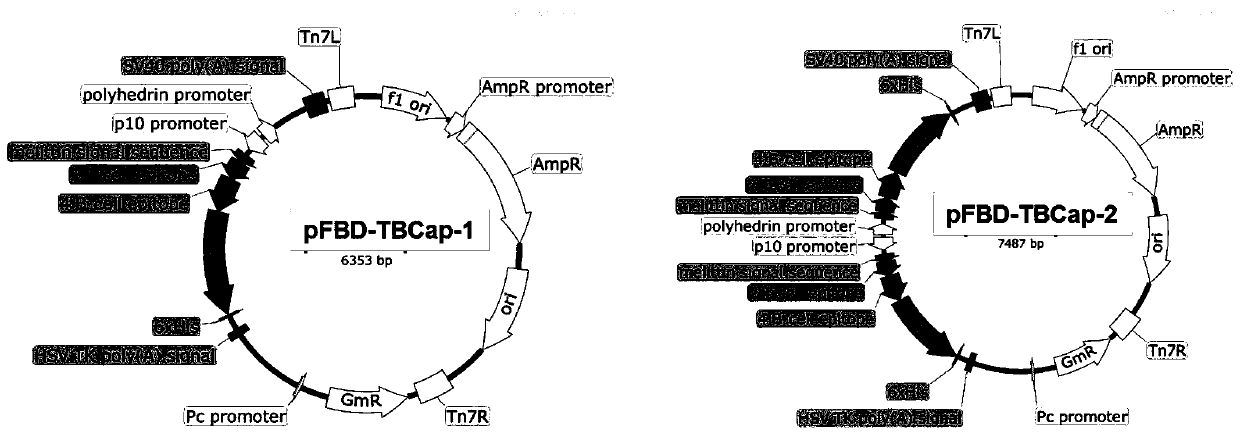
[0056] Using the complete gene sequence of PCV2 LG strain (HM038034.1) recorded in GenBank as a template, the Cap / Rep linear epitope was introduced into the PCV2 cap protein. Peptides with good antigenicity and high specificity, namely the 81-100, 201-220 on the Rep protein and the 61-85, 113-131, 169-180, 192-202 epitopes on the Cap protein, And determine the tandem sequence between the dominant antigenic epitopes, and introduce the honeybee melittin signal peptide sequence (HBM) in order to achieve secreted expression, design a base sequence encoding 6 histidines before the stop codon, and finally The nucleotide sequence of the recombinant protein PCV2-TBCap was designed, and the nucleotide sequence was codon-optimized for insect cells, and then sent to Shanghai Sangon Bioengineering Co., Ltd. for synthesis. The nucleotide se...
Embodiment 2
[0076] Example 2 Obtaining of recombinant baculovirus and optimization of recombinant protein expression conditions
[0077] (1) Obtaining recombinant bacmid
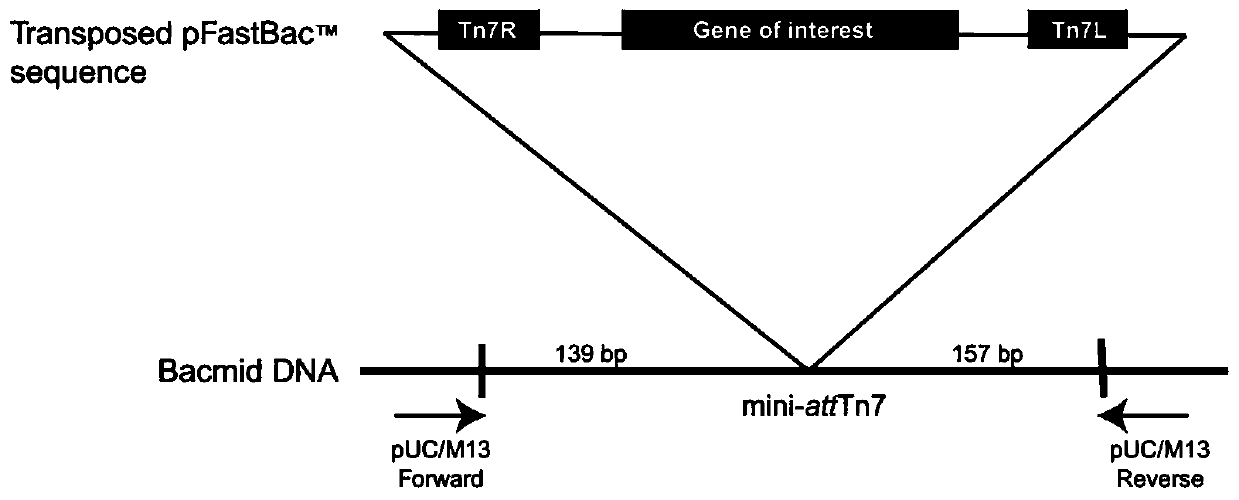
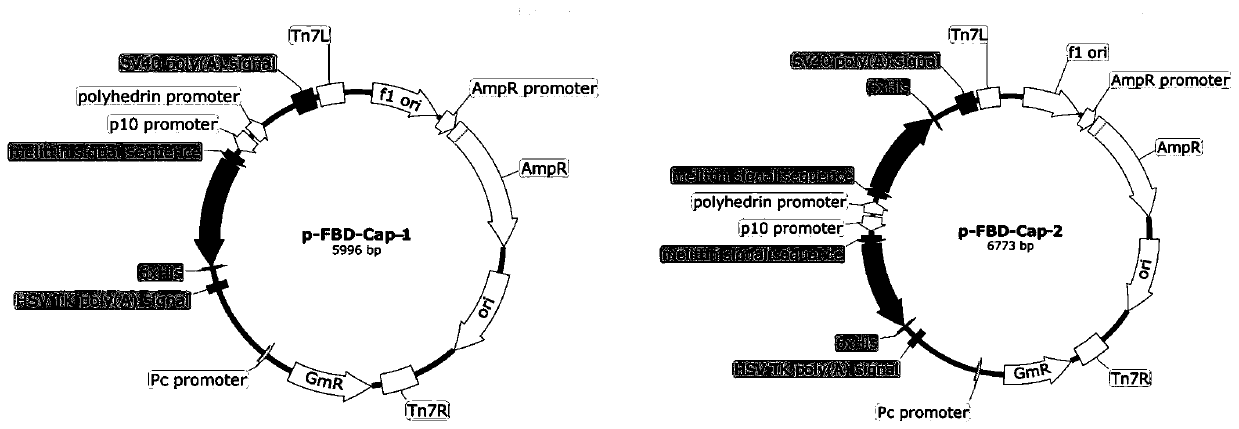
[0078] Transform the successfully identified recombinant baculovirus transfer vectors pFBD-Cap-1, pFBD-Cap-2, pFBD-TBCap-1, and pFBD-TBCap-2 into DH10Bac-competent cells respectively, and gently blow and mix with a pipette gun, and place on ice ② Take the competent cells out of the ice bath, place them in a water bath at 42°C for 45 seconds, and then take them out and incubate them in the ice bath for 2 minutes; ③Add 900 μL of SOC medium to the EP tube, and incubate at 37°C Bacteria culture shaker 200rpm shaking culture transposition 4h; ④ use SOC medium to gradiently dilute the transformed bacteria (10 -1 , 10 -2 , 10 -3 ), respectively draw 100 μL of the above-mentioned diluted gradient transformed bacteria and spread them on the solid medium of LB (Gen, Kan, Tet, IPTG, X-Gel, use concentration according to Bac-to-...
Embodiment 3
[0111] The immune efficacy experiment of the subunit vaccine prepared by embodiment 3 recombinant protein
[0112] (1) Preparation of PCV2 subunit vaccine
[0113] Inoculate the third-generation recombinant baculovirus solution Ac-Cap-2 and Ac-TBCap-2 at a concentration of 2×10 6 Cells / mL High Five suspension cells were placed in a constant temperature shaker at 27°C at 120rpm to shake the cells, and the cell pellets were collected by centrifugation according to the optimal harvest time, and an appropriate amount of PBS was added to resuspend the cells, placed on ice, and the cells were ultrasonically broken. , and then centrifuged at 11,000 rpm for 15 min at 4°C. The collected supernatant is the expressed immune antigen solution.
[0114] Adjust the concentration of the recombinant protein to 1000 μg per ml, and emulsify the recombinant protein Cap and TBCap of this concentration with the ISA201VG adjuvant at a ratio of 1:1 to prepare a PCV2 subunit vaccine containing 100 μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com