CHO cell strain modified based on CRISPR/Cas9 gene editing and preparation method thereof
A gene editing and cell line technology, applied in the field of gene editing, can solve the problems of high price and complicated design, and achieve the effects of strong anti-apoptotic ability, high catalytic disulfide bond efficiency, and high protein expression quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
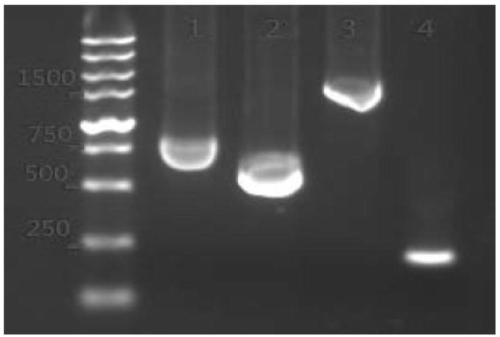
[0030] Example 1. Design, construction and functional verification of sgRNA-Cas9 and expression cassette based on CHO-K1 cells
[0031] Reagents and kits: Premix Taq DNA polymerase, Pyrobest DNA polymerase, restriction endonucleases (Hind III, BamH I), DNA A-Tailing Kit, pMDTM19-T Vector Cloning Kit, DNAmarker were purchased from Bao Biological Engineering Co., Ltd. (Dalian). Endonuclease Bbs I and T4 DNA ligase were purchased from NEB. Plasmid Extraction Kit, Blood / Cell, Tissue Genomic DNA Extraction Kit, EasyGene Rapid Recombination Cloning Kit, DNA Gel Recovery Kit and UniversalDNA Purification and Recovery Kit were purchased from Tiangen. Lipofectamine 2000 was purchased from Thermo Fisher Scientific. Tryptone and yeast extract were purchased from Oxoid Company (UK). Ampicillin was purchased from Sangon Bioengineering Co., Ltd. (Shanghai). F-12 and Opti-MEM medium, trypsin were purchased from Gibco. Penicillin-streptomycin (100×) was purchased from Solarbio. FBS was p...
Embodiment 2
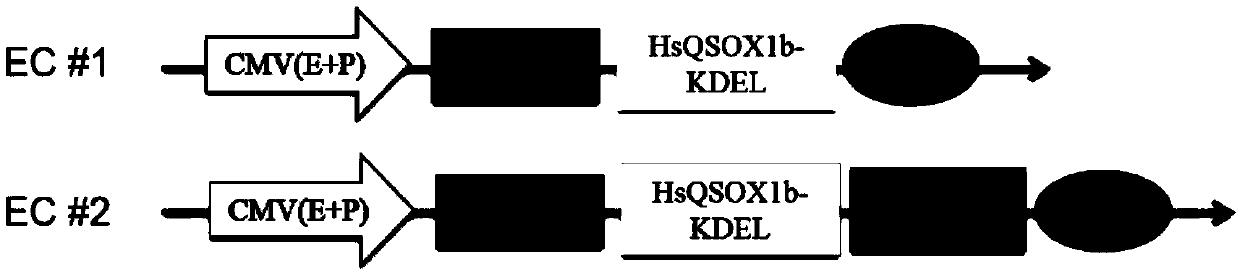
[0078] Example 2. Establishment of monoclonal stable cell lines overexpressing HsQSOX1b and Survivin
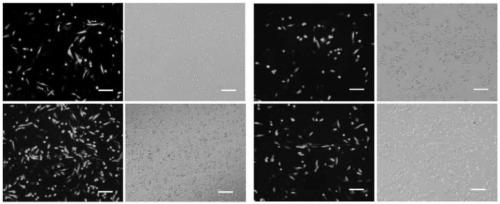
[0079] Reagents and kits: PMSF and BCAProtein Assay kit were purchased from Shanghai Sangong. HRP-conjogated goat anti-mouse IgG (H+L), HRP-conjogated goat anti rabbit, Anti-βactin antibody and Anti-QSOX1 antibody were purchased from Proteintech. Pro-light HRPchemiluminescent kit was purchased in Tiangen, Beijing. ER-Tracker Red and Hoechst 33342 dye were purchased from Thermo Fisher Scientific. Other biochemical reagents belong to domestic conventional analytical reagents.
[0080] Cells, strains and plasmids: recombinant plasmids sgRNA1-QSOX1-pX458(Q1), sgRNA2-QSOX1-pX458(Q2), sgRNA1-BIRC5-pX458(B1) and sgRNA2-BIRC5-pX458(B2) were constructed for the above stages of the present invention . E. coli DH5α (Invirogen, USA) was used as a plasmid amplification strain; cell line CHO-K1 cells (Cell Bank of Chinese Academy of Sciences).
[0081] Reagent preparation:
[0082] 5...
Embodiment 3
[0097] Example 3. Preliminary study on the quality control of intracellular protein in monoclonal strains overexpressing HsQSOX1b and Survivin
[0098] Reagents and kits: PrimeScript TM RT Master Mix, Premix Ex Taq and restriction endonucleases (Hind III, BamH I) were purchased from TaKaRa. Annexin V-PE apoptosis detection kit and GSHand GSSG Assay Kit were purchased from Beyotime. Gaussia Luciferase Assay Kit was purchased from NEB. PI Staining Solution was purchased from Yeasen. Other biochemical reagents belong to domestic conventional analytical reagents.
[0099] Cells and plasmids: Cells: monoclonal strain EC#1-C1 stably expressing HsQSOX1b-KDEL and monoclonal strain EC#2-B6 stably expressing HsQSOX1b-KDEL and Survivin; plasmids: pGluc-Basic, and pCDNA3.1.
[0100] Reagent preparation:
[0101] Preparation of 5mg / mL MTT stock solution: Accurately weigh 250mg MTT and dissolve it in 50mL of PBS, then filter and sterilize with a 0.22μm sterile filter membrane, aliquo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com