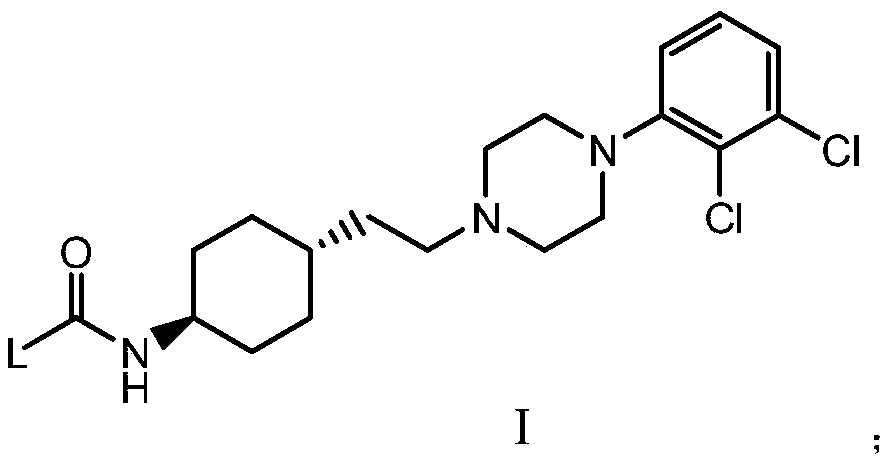
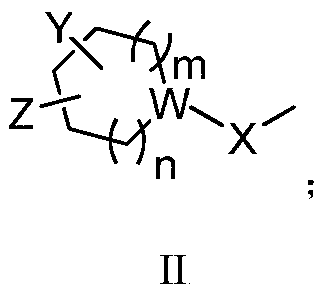
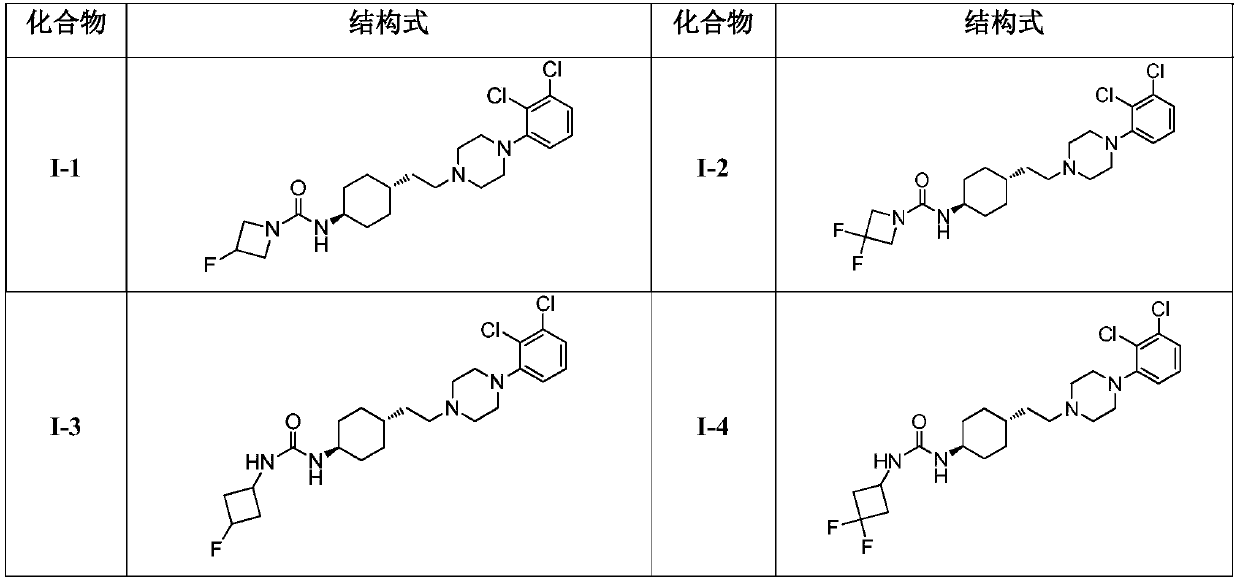
Cyclohexane amine D3/D2 receptor partial agonist
A cyclohexane amine and agonist technology, applied in the field of biomedicine, can solve problems such as increased risk of death and slurred speech, and achieve the effects of low clinical dose, enhanced mutual binding ability, and excellent PK properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0059] Reference example 1: Synthetic route of general intermediate 5.
[0060]
[0061] Steps:
[0062] Step 1: Synthesis of Intermediate 2.
[0063] Add compound 1 (2.43g, 10mmol) and triethylamine (2g, 20mmol) into dichloromethane (30mL), cool down to an internal temperature of 0-5°C, and add methanesulfonyl chloride (1.1g, 10mmol) dropwise under stirring. Chloromethane (10 mL) solution, after the dropwise addition, was raised to room temperature and reacted for 3 hours; after the reaction was completed, the solvent was concentrated to dryness to obtain the crude intermediate 2.
[0064] Step 2: Synthesis of Intermediate 4
[0065] In step 1, the crude intermediate 2 (10 mmol) was concentrated and added to acetonitrile (20 mL), then potassium carbonate (2.76 g, 20 mmol) and compound 3 (2.54 g, 11 mmol) were added, the temperature was raised to reflux and the reaction was stirred for 8 h. After the reaction was completed, the temperature was lowered to room temperature...
Embodiment 1
[0068] Embodiment 1: the synthesis of I-1
[0069] synthetic route:
[0070]
[0071] Steps:
[0072] Add compound 5 (500mg, 1.27mmol), IA-1 (142mg, 1.27mmol), triethylamine (643mg, 6.36mmol) into dichloromethane (5mL), cool the system to an internal temperature of 0-5°C, and add triphosgene (453 mg, 1.53 mmol). After the addition was completed, the reaction was stirred at room temperature for 4 h, and the reaction was monitored by TLC to complete. Dichloromethane (10mL) and water (10mL) were added to the reaction solution for extraction, the organic layer was washed with saturated brine (10mL), the organic layer was concentrated to dryness, and the residue was purified by silica gel column chromatography (the mobile phase was dichloromethane / methanol: 30 / 1), the product I-1 (250 mg, yield 43%) was obtained as a pale white solid. MS Calcd.: 457.4, MS Found: 458.1 [M+H] + . 1 H NMR (400MHz, CD 3 OD): δ=7.23(m,2H),6.81(m,1H),4.13-3.89(m,5H),3.67(m,1H),3.47(m,8H),2.49(...
Embodiment 2
[0073] The synthesis of embodiment 2:1-2
[0074] synthetic route:
[0075]
[0076] Steps:
[0077] Refer to Example 1 for the operation steps and purification method, and the yield is 50%. MS Calcd.: 475.4, MS Found: 476.1 [M+H] + . 1 H NMR (400MHz, CD 3 OD): δ=7.25(m,2H),6.83(m,1H),4.16(t,J=15.6Hz,4H),3.65(m,1H),3.47(m,8H),2.48(t,J =5.6Hz, 2H), 1.77(m, 2H), 1.55-1.37(m, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com