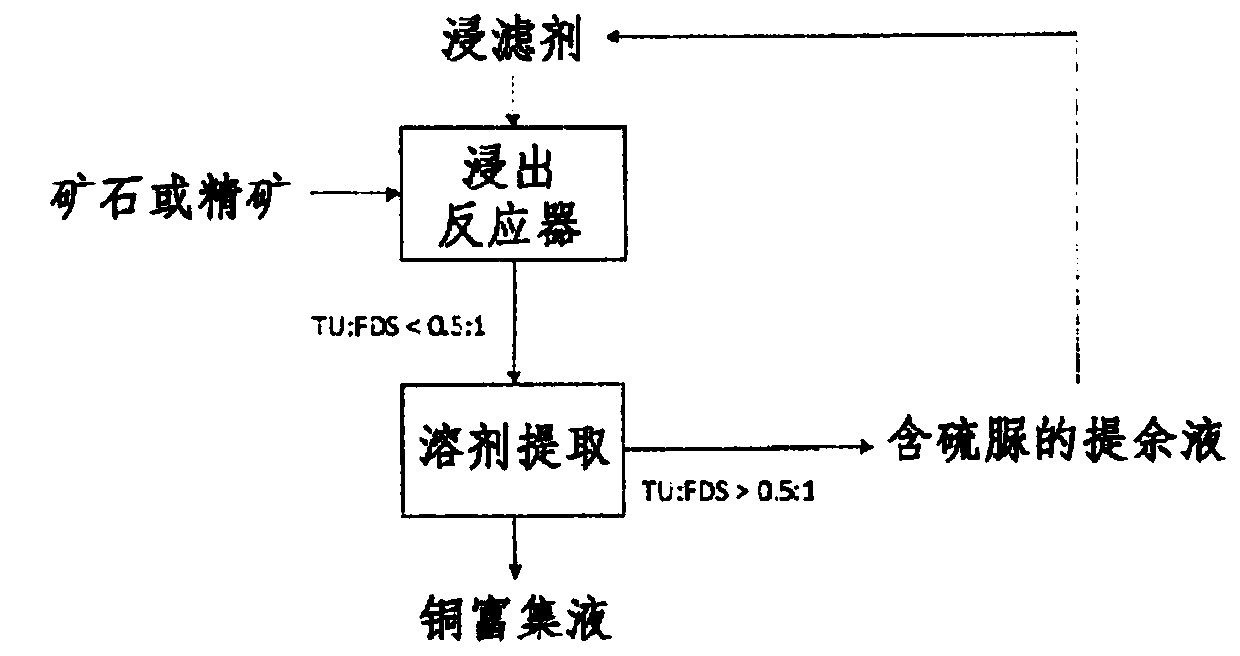
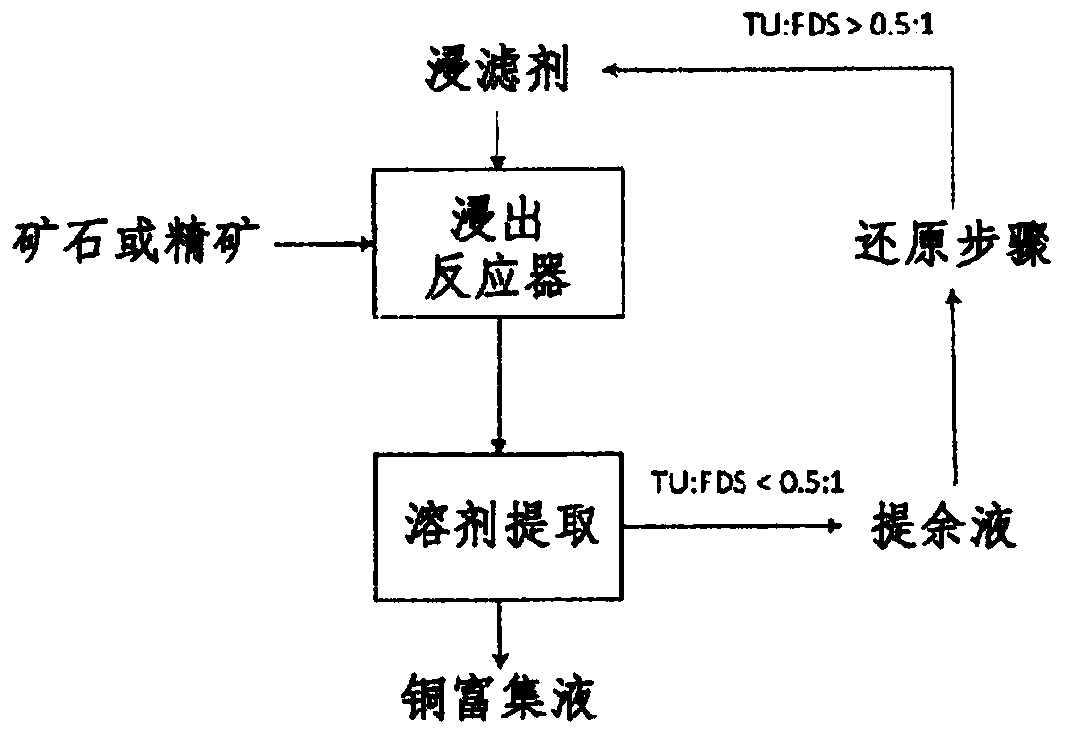
Process for leaching metal sulfides with reagents having thiocarbonyl functional groups
A thiocarbonyl functional, metal sulfide technology, applied in the preparation of sulfide/polysulfide, the preparation of cadmium compounds, cadmium sulfide, etc., can solve the problem of not showing the recovery of copper and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Example 1 Extraction of copper from chalcopyrite using thiourea
Embodiment 11
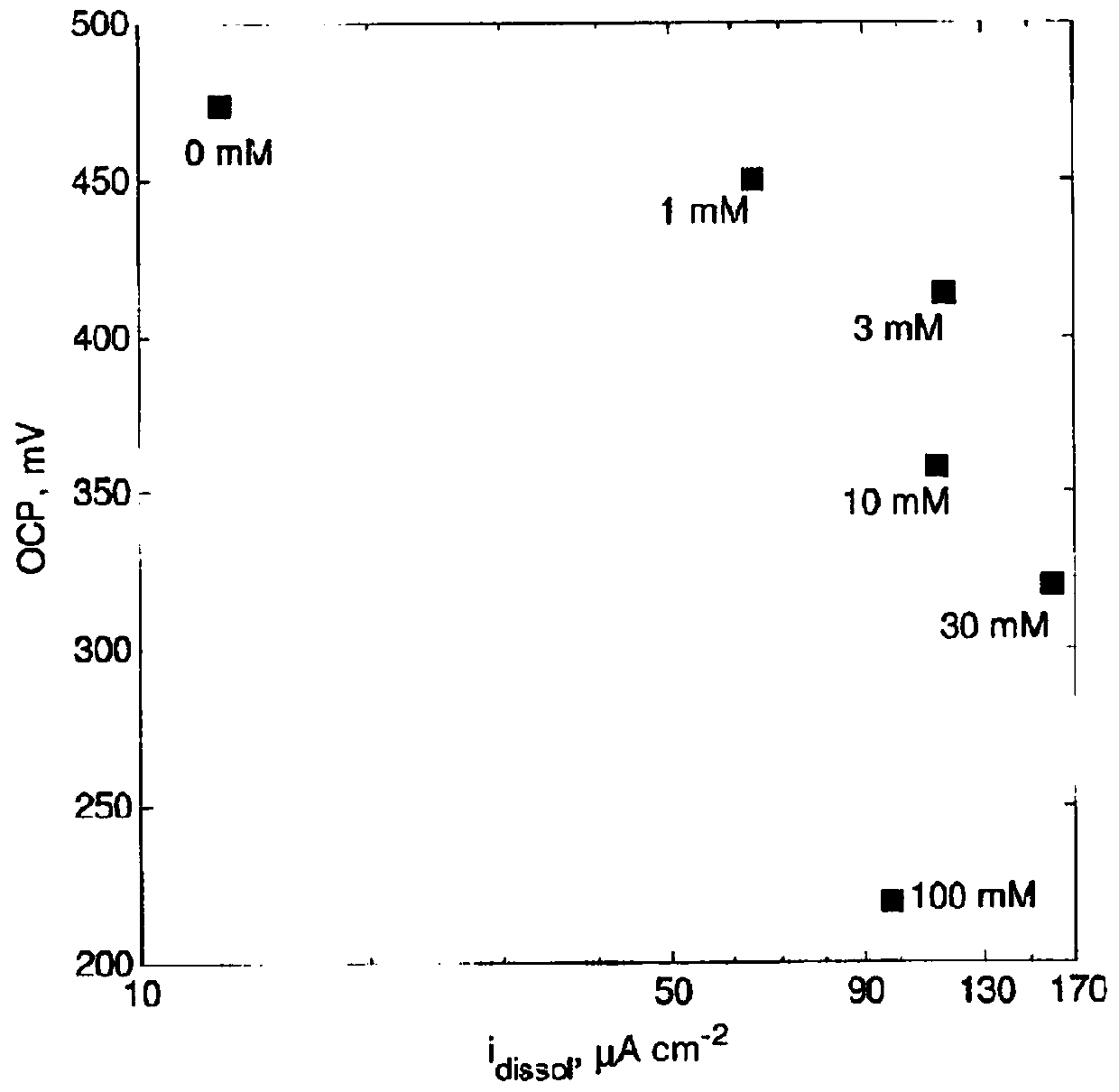
[0193] The effect of Tu on the electrochemical behavior of chalcopyrite electrodes was investigated in a conventional 3-electrode glass-jacketed cell. CuFeS 2 The electrode was used as a working electrode, a saturated calomel electrode (SCE) was used as a reference, and a graphite rod was used as a counter electrode. Use 600 and 1200 grit carbide sandpaper for CuFeS 2 The electrodes are polished. All experiments were performed at 25°C using a temperature-controlled water bath. The composition of the electrolyte is 500mM H 2 SO 4 , 20mM Fe 2 SO 4 and 0-100 mM Tu. Before starting any measurements, the solution was decontaminated with N 2 Bubble for 30 min to reduce dissolved O 2 concentration. The open circuit potential (OCP) was recorded until a change of no more than 0.1 mV / min was observed. After a stable OCP value was observed, electrochemical impedance spectroscopy (EIS) was performed at OCP using 5 mV a.c. sinusoidal perturbation from 10 kHz to 10 mHz. Self-lin...
Embodiment 12
[0198] Figure 4 is a bar graph showing the effect of initial Tu or FDS concentration on the electrochemical dissolution of chalcopyrite electrodes in sulfuric acid solution at pH 2 at 25°C. A concentration of 10 mM Tu in the leach solution resulted in a 6-fold increase in dissolution rate compared to no Tu; a concentration of 5 mM FDS resulted in a 6-fold increase relative to 10 mM Tu. A concentration of 10 mM Tu in the leaching solution also containing 40 mM Fe(III) resulted in a 30-fold increase in the dissolution rate compared to 40 mM Fe(III) alone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com