Double-condensed-ring naphthopyran photochromic compound and preparation method thereof
A photochromic and compound technology, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve the problems of insufficient light response, low color rate of chromophores, and limited application range, etc., to achieve rapid response, The effect of high color ratio and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
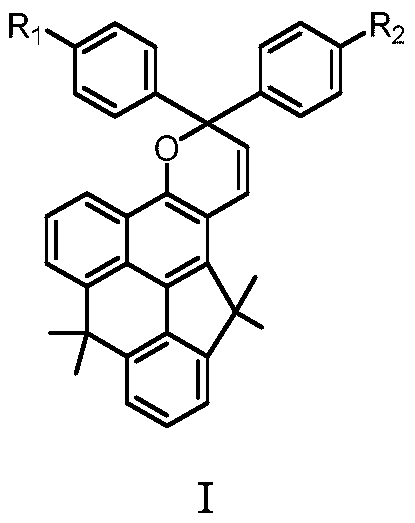
[0032] The preparation of embodiment 1 photochromic compound Ia
[0033] The first step: the preparation of compound M1, the reaction formula is as follows:
[0034]
[0035] In a 100mL three-necked flask, add 2.4g (8.45mmol) of 4-methoxynaphthalene-1-ylboronic acid pinacol ester, 1.75g (6.4mmol) of dimethyl 2-bromoisophthalate, tetrakistriphenylphosphine Palladium 890mg (0.768mmol), anhydrous sodium carbonate 3.9g (36.86mmol), the device was evacuated and filled with nitrogen. Inject 1,4-dioxane / water (V 二氧六环 / V 水 =5:2) mixed solvent 56mL, reacted at 80°C for 12h, and stopped the reaction after the disappearance of the raw material point as monitored by TLC. Cool to room temperature, filter with celite, wash the organic phase three times with water, and extract with ethyl acetate. Combined organic layers, anhydrous MgSO 4 dry. The crude product obtained by distillation under reduced pressure was separated by column chromatography (petroleum ether / ethyl acetate=20:1...
Embodiment 2
[0052] The photochromic property test of embodiment 2 compound Ia
[0053] Take compound Ia, configure 8×10 -5 mol / L ethyl acetate solution. The solution was colorless before light irradiation, and the solution changed from colorless to purple rapidly when irradiated with ultraviolet light.
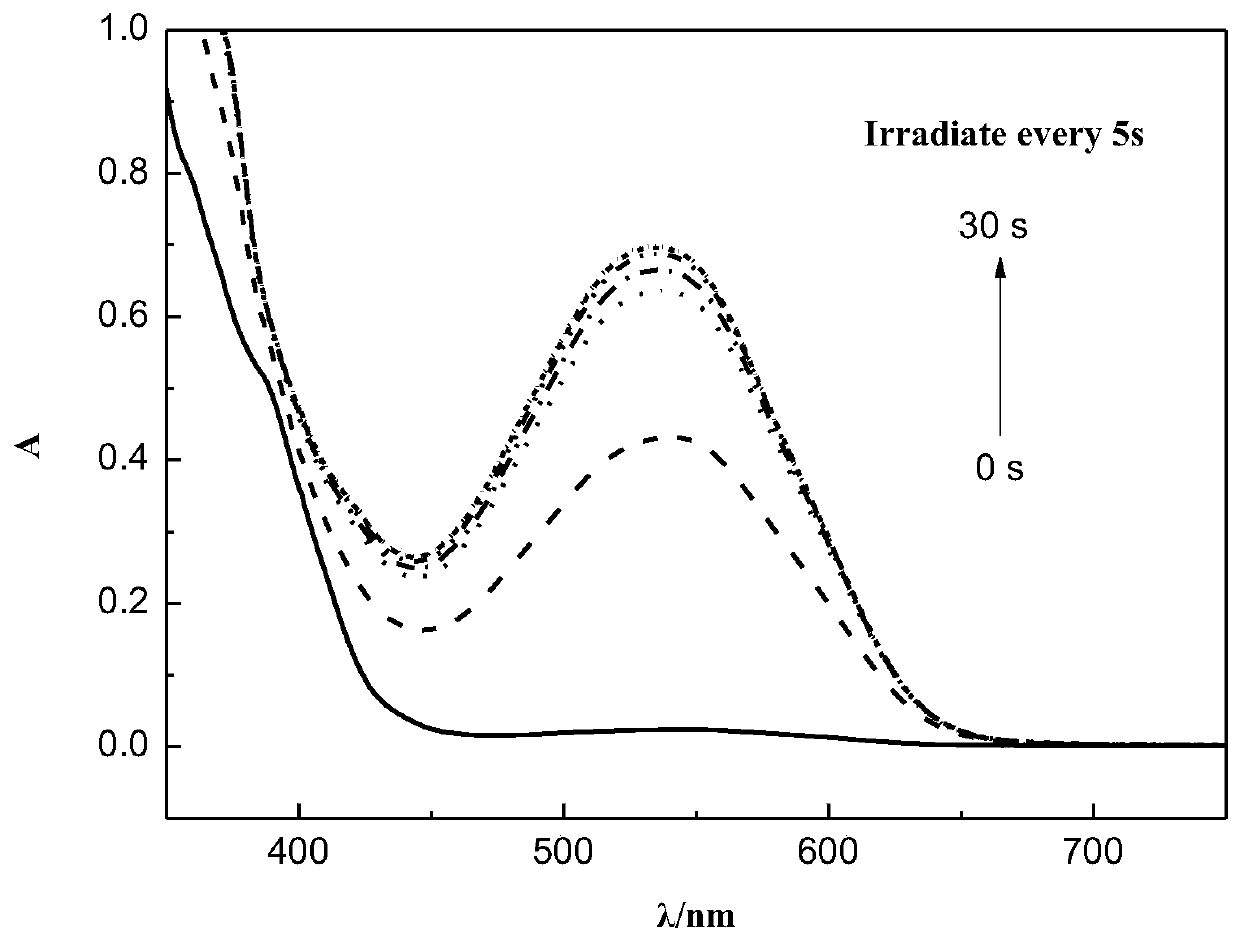
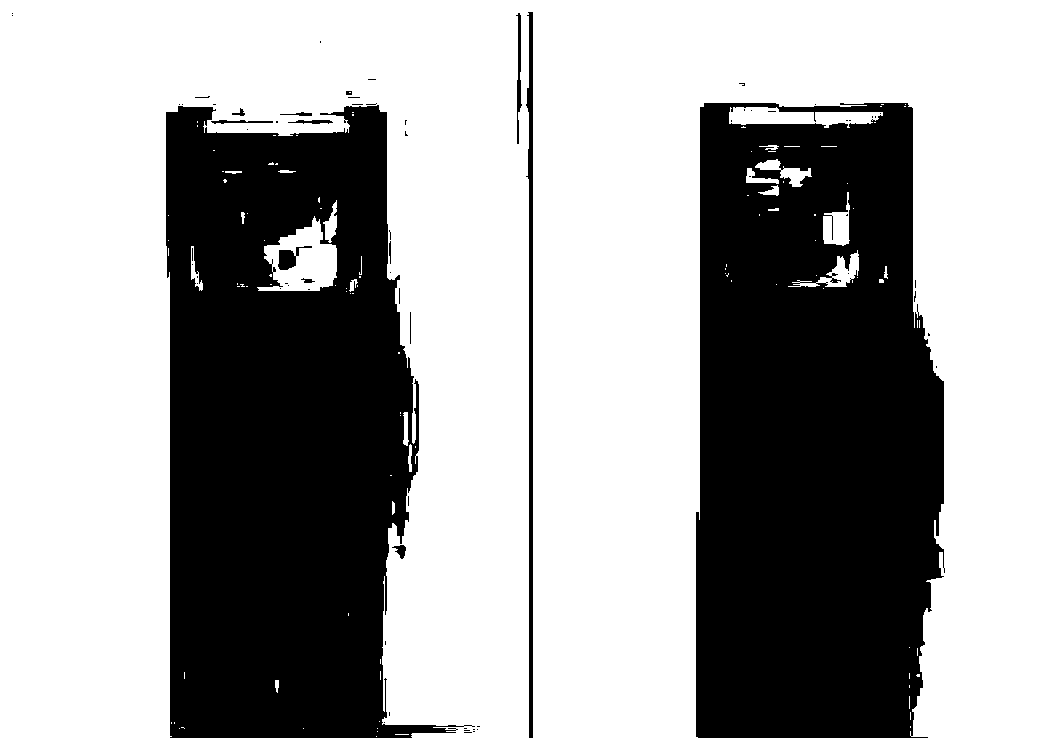
[0054] attached figure 1 It is the discoloration phenomenon of the toluene solution of compound Ia irradiated with ultraviolet light for 10 seconds.
[0055] attached figure 2 is compound Ia ethyl acetate solution (8×10 -5 mol / L) UV-Vis absorption spectrum when illuminated. Depend on figure 2 Calculated by the fitting formula, when the absorbance reaches half of the saturation value, the time is 3.6s, and when it reaches full saturation, the time is 12.6s, indicating that the compound changes color rapidly. Dilute to 8 x 10 in concentration -5 When mol / L, its saturated absorbance still reaches 0.7, shows that the color rate of compound Ia is high.
[0056] The ethyl acetate sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com