Application of phenylboronic acid in preparing nuclear-targeted boron capture agent
A technology for coupling boron phenylboronic acid and phenylboronic acid, which is applied in the direction of active ingredients of boron compounds, medical preparations of non-active ingredients, antineoplastic drugs, etc., can solve the problem of unsatisfactory tumor targeting effect and achieve biocompatibility good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
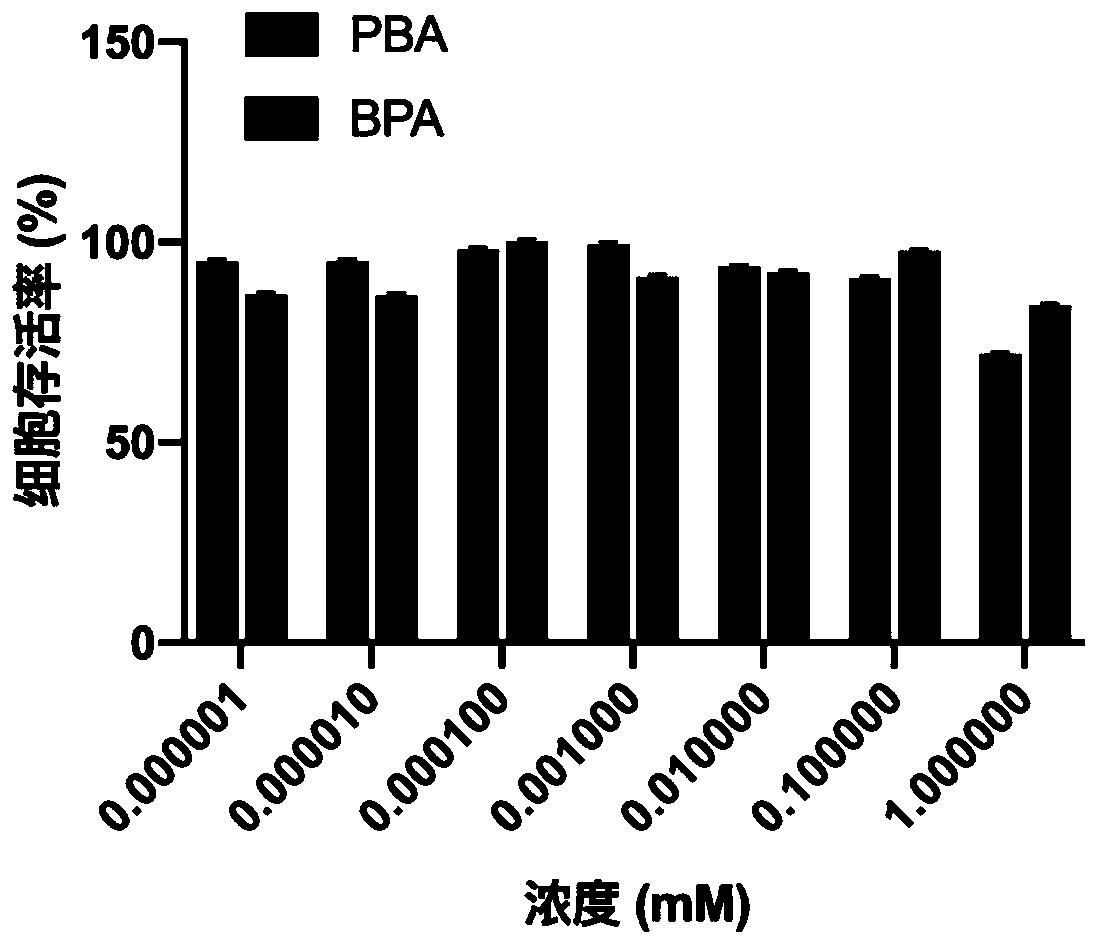
[0035] Example 1 Cytotoxicity experiment of small molecule phenylboronic acid (PBA) boron capture agent on B16F10 cells
[0036] The B16F10 cells in the logarithmic growth phase were inoculated in a 96-well plate, and the number of cells per well was 5×10 3 , at 37°C, 5% CO 2 After culturing in the cell incubator for 24 hours, remove the culture medium, add 200 μL of culture medium containing PBA or 4-boron-L-phenylalanine (BPA) to each well, set up 7 equal concentration gradients, and place in the cell incubator to continue incubation Remove the culture medium after 48 hours, add 100 μL of culture medium containing 10% CCK8 to each well, continue to cultivate for 4 hours, shake on the shaker for 2 minutes, use a microplate reader to measure the OD value at a wavelength of 450 nm, and use the formula to calculate the cell survival rate to evaluate the cells toxicity:
[0037]
[0038] Such as figure 1 As shown, BPA is a safe boron capture agent that has been clinically u...
Embodiment 2
[0039] Embodiment 2 small molecule phenylboronic acid (PBA) boron capture agent in B16F10 nucleoplasm fluorescence distribution
[0040] Amino-modified phenylboronic acid (4-aminophenylboronic acid) was dissolved in water, added with 1 equivalent of rhodamine B and EDCI catalyst to react overnight, and then dialyzed for 12 hours to obtain rhodamine (RHB) fluorescently labeled PBA (PBA-RHB). B16F10 cells were incubated with RHB and PBA-RHB solutions (both at a concentration of 5 μg / ml) for 2 hours, 6 hours, and 12 hours, respectively. After washing, the cells were fixed. DAPI staining marked the nuclei, and the fluorescence distribution of nuclei and cytoplasm was evaluated under a confocal microscope. Condition.
[0041] Such as figure 2As shown, the PBA-RHB red fluorescence was obviously distributed in the nucleus after 2 hours of incubation, and part of the PBA-RHB red fluorescence was still in the nucleus after 6 hours ( image 3 ), but after 12 hours there was little PB...
Embodiment 3
[0042] Embodiment 3 Small molecule phenylboronic acid (PBA) boron capture agent is distributed in B16F10 cell nucleoplasm
[0043] Prepare PBA with a boron concentration of 1 μg / mL and incubate B16F10 cells for 4 hours, and then use ICP-MS to evaluate the amount of boron uptake per million cells (n=3). Methods Boron uptake per million nuclei was assessed (n=3).
[0044] Such as Figure 5 As shown, B16F10 cells have more than 20ng B / 10 to PBA preparation 6 Cells uptake, more than half of the boron is located in the nucleus, the effect of the preparation meets the expected design, and is suitable for further modification or encapsulation in nano preparations to achieve tumor-targeted delivery in vivo.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com