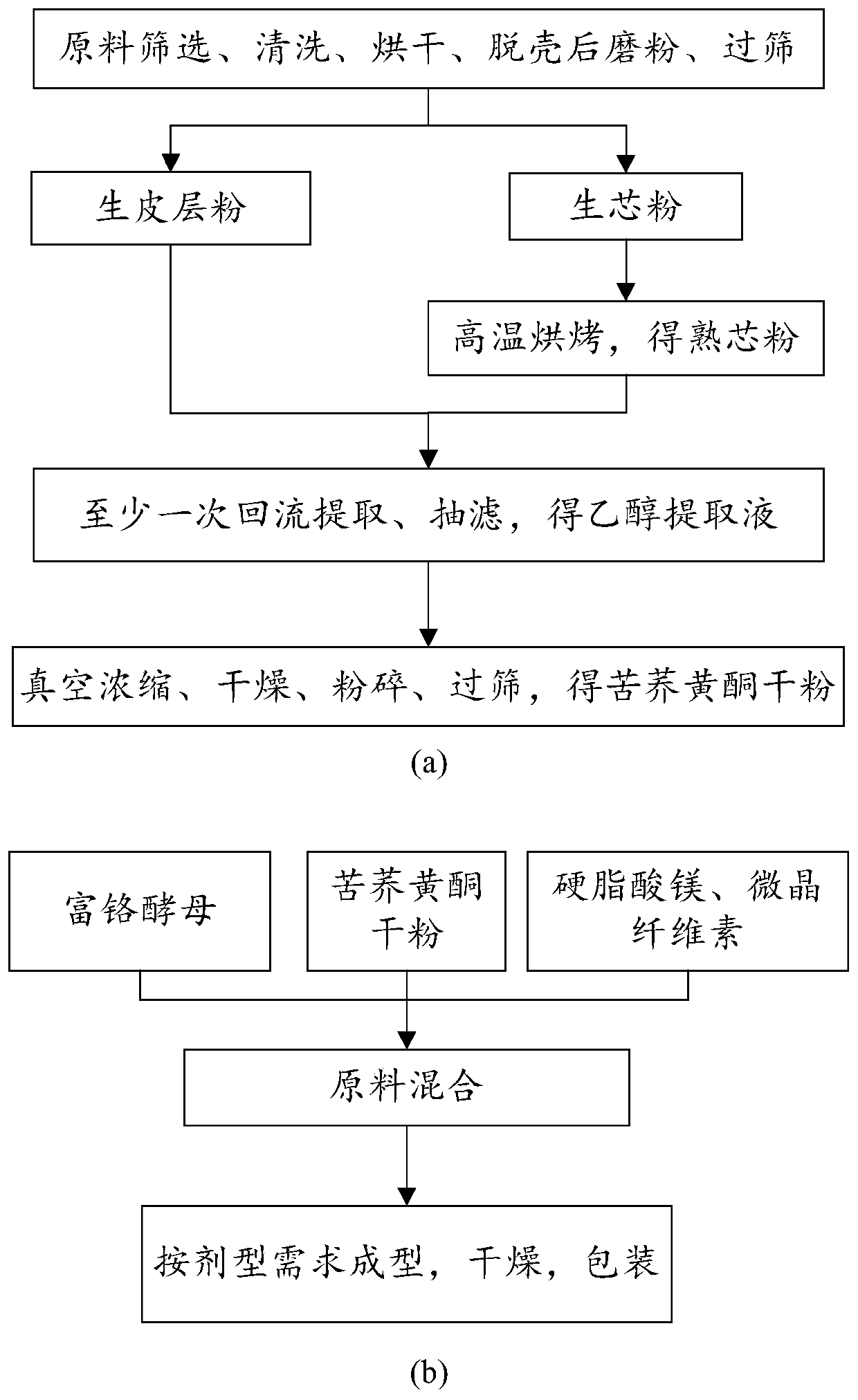
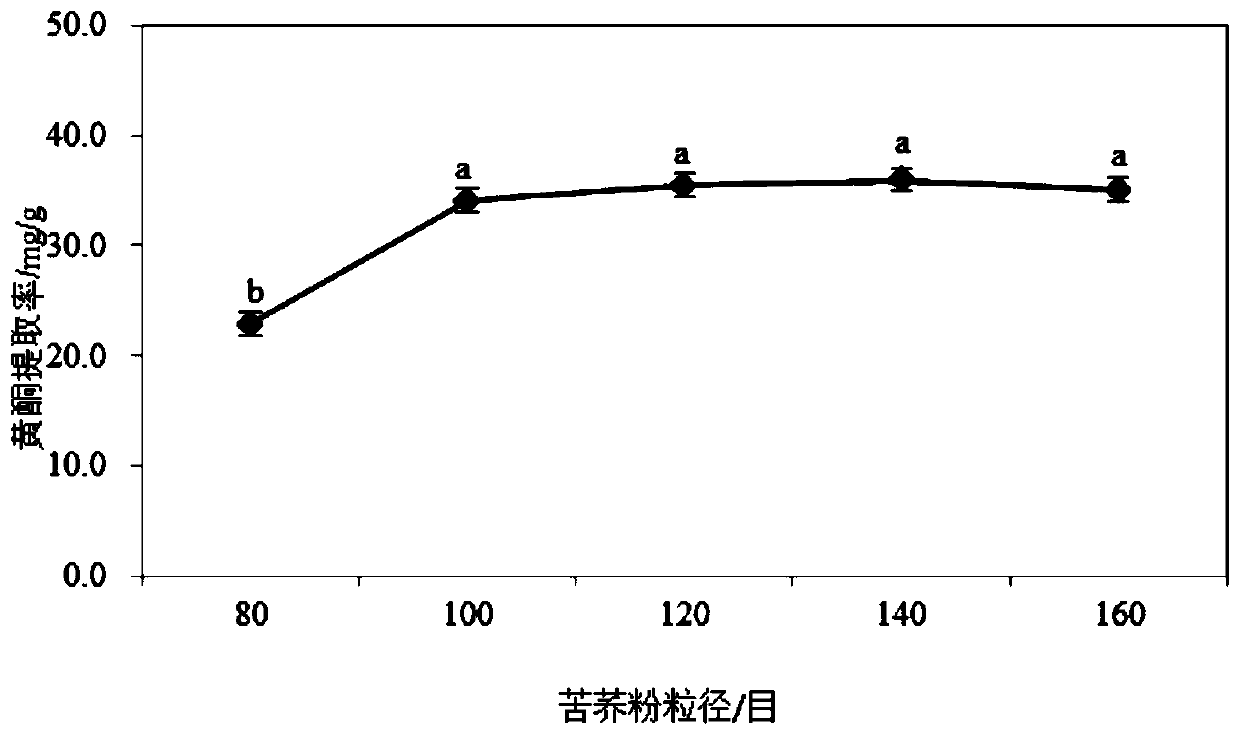
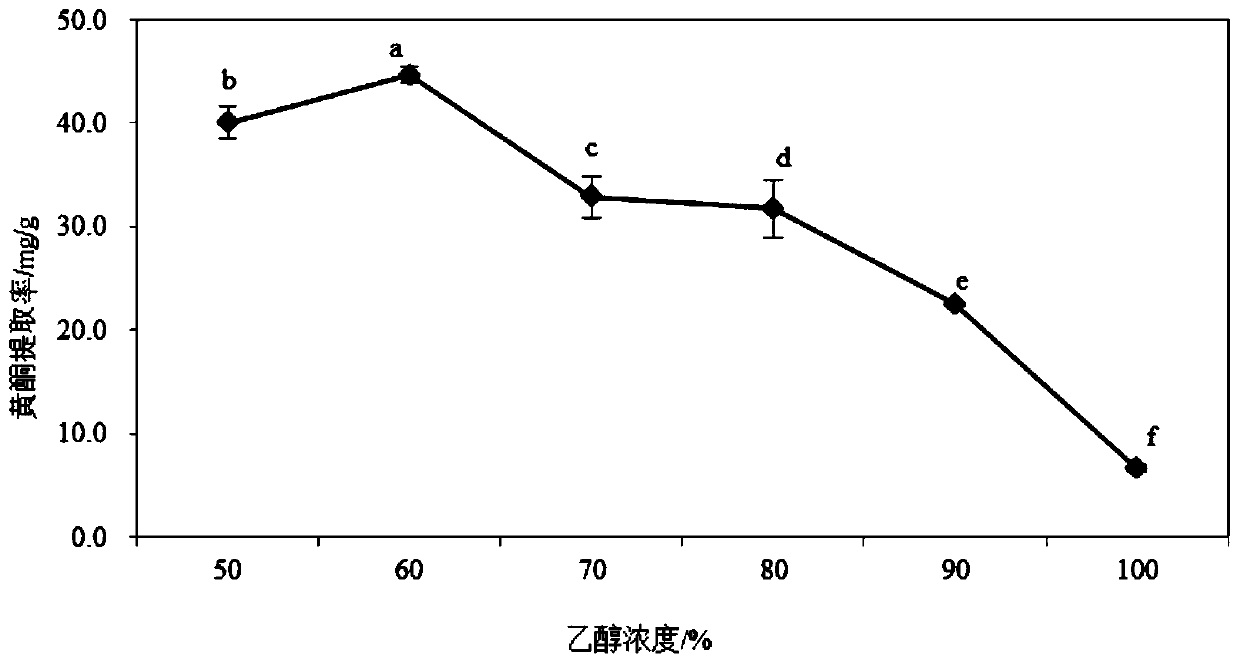
Tartary buckwheat flavone dry powder and use of tartary buckwheat flavone dry powder in preparing health care products with auxiliary hypoglycemic function
A technology of tartary buckwheat flavonoids and health products, which is applied in the field of food processing, can solve the problems of low content of tartary buckwheat flavonoids and low utilization rate of raw materials, and achieve the effects of small toxic and side effects, mild effect and great market potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1 Hyperglycemia model animal hypoglycemic experiment
[0094] The hyperglycemia model animals were selected and grouped according to the blood glucose level after fasting for 3 to 5 hours, and a model control group and 3 dose groups were randomly selected (the difference between the groups was not greater than 1.1 mmol / L). The dose group was given different concentrations of test samples, and the model control group was given solvent. For 30 consecutive days, the fasting blood glucose was measured (fasting was the same as before the experiment), and the blood glucose and blood glucose reduction percentages of animals in each group were compared.
[0095]
Embodiment 2
[0096] Example 2 Glucose tolerance experiment of hyperglycemia model animals
[0097] Dose grouping and test sample administration time are the same as 1.4.2. Animals in each group were fasted for 3 to 5 hours, and blood glucose levels were measured before glucose or medical starch was given (that is, 0 hours). The dose group was given different concentrations of test samples, and the model control group was given the same volume of solvent. After 15 to 20 minutes, each group Oral administration of glucose 2.0g / kg BW or medical starch 3-5g / kg BW, measure the blood glucose values of each group at 0.5 and 2 hours after glucose administration or 1 and 2 hours after administration of medical starch, observe the model control group and Changes in the blood glucose value and the area under the blood glucose curve at each time point (0, 0.5, 2 hours) after the test sample group was given glucose or medical starch.
[0098]
Embodiment 3
[0099] Embodiment 3 Normal group mice body weight, blood sugar and glucose tolerance test data statistics
[0100] Table 10: Effect of Black Tartary Buckwheat Hypoglycemic Capsules on Body Weight of Normal Mice (n±s)
[0101] group Before gavage 2 weeks after gavage 3 weeks after gavage 4 weeks after gavage normal blank group 36.05±3.56 36.40±4.07 38.30±4.49 39.70±5.02 normal high dose group 34.49±2.63 34.66±2.38 36.82±2.69 37.42±2.58
[0102] It can be seen from Table 10 that, compared with the blank control group, there was no significant difference in the body weight of the normal mouse high-dose group in the early stage, middle stage and late stage of the experiment (P>0.05).
[0103] Table 11: Effect of Black Tartary Buckwheat Hypoglycemic Capsules on Fasting Blood Glucose in Normal Mice (n±s)
[0104]
[0105] It can be seen from Table 11 that, compared with the blank control group, there was no significant difference in fastin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com