1-butyl-3-methylimidazole-tri(hexafluoroacetylacetone) cobalt (II) complex magnetic ionic liquid, and preparation method and application thereof
A technology of hexafluoroacetylacetone and magnetic ionic liquid, applied in the field of preparation, 1-butyl-3-methylimidazolium triple cobalt magnetic ionic liquid, can solve separation difficulties, low recovery rate, unsuitable for liquid chromatography, etc. problems, to achieve the effect of expanding the scope of application, simple preparation method, and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
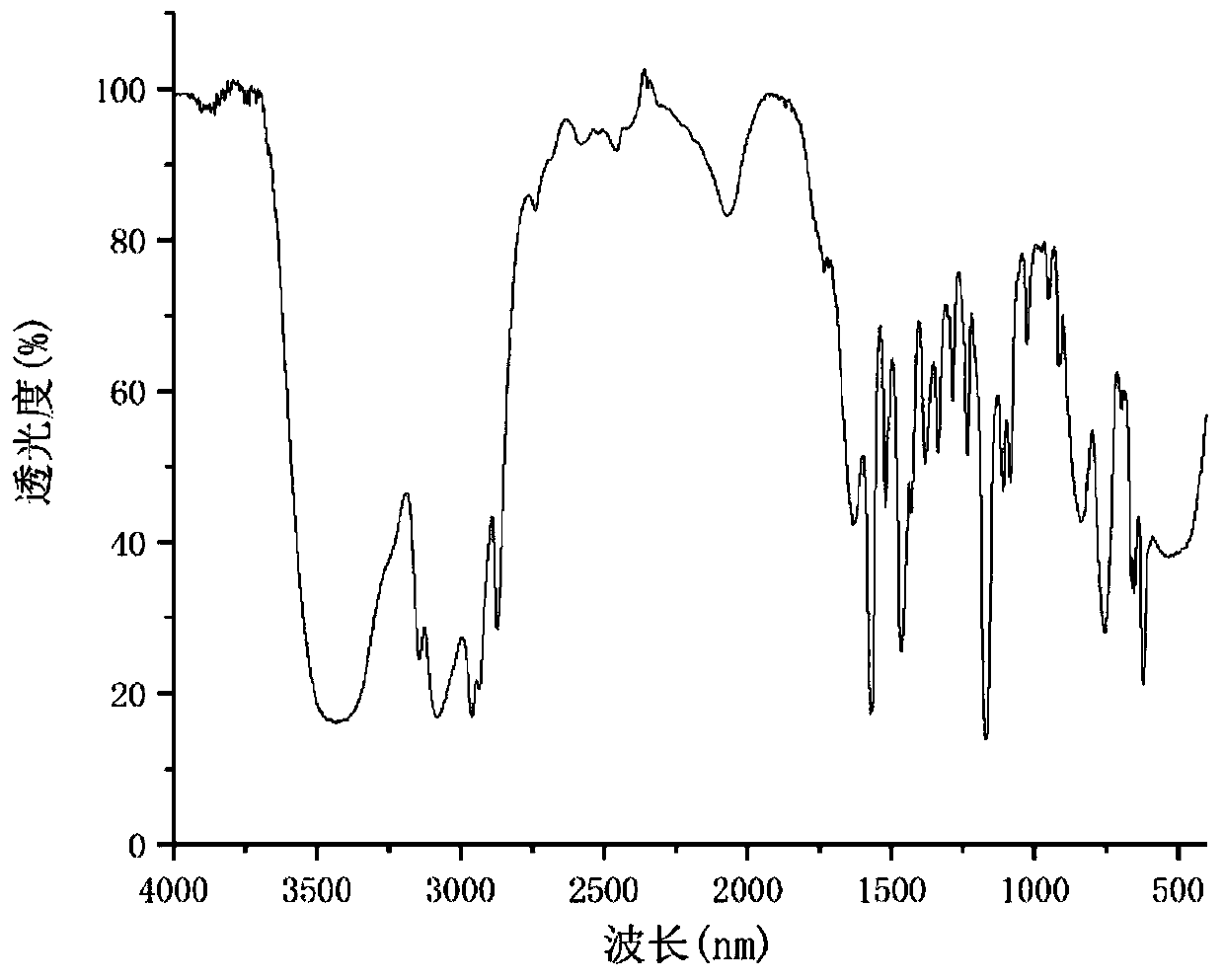
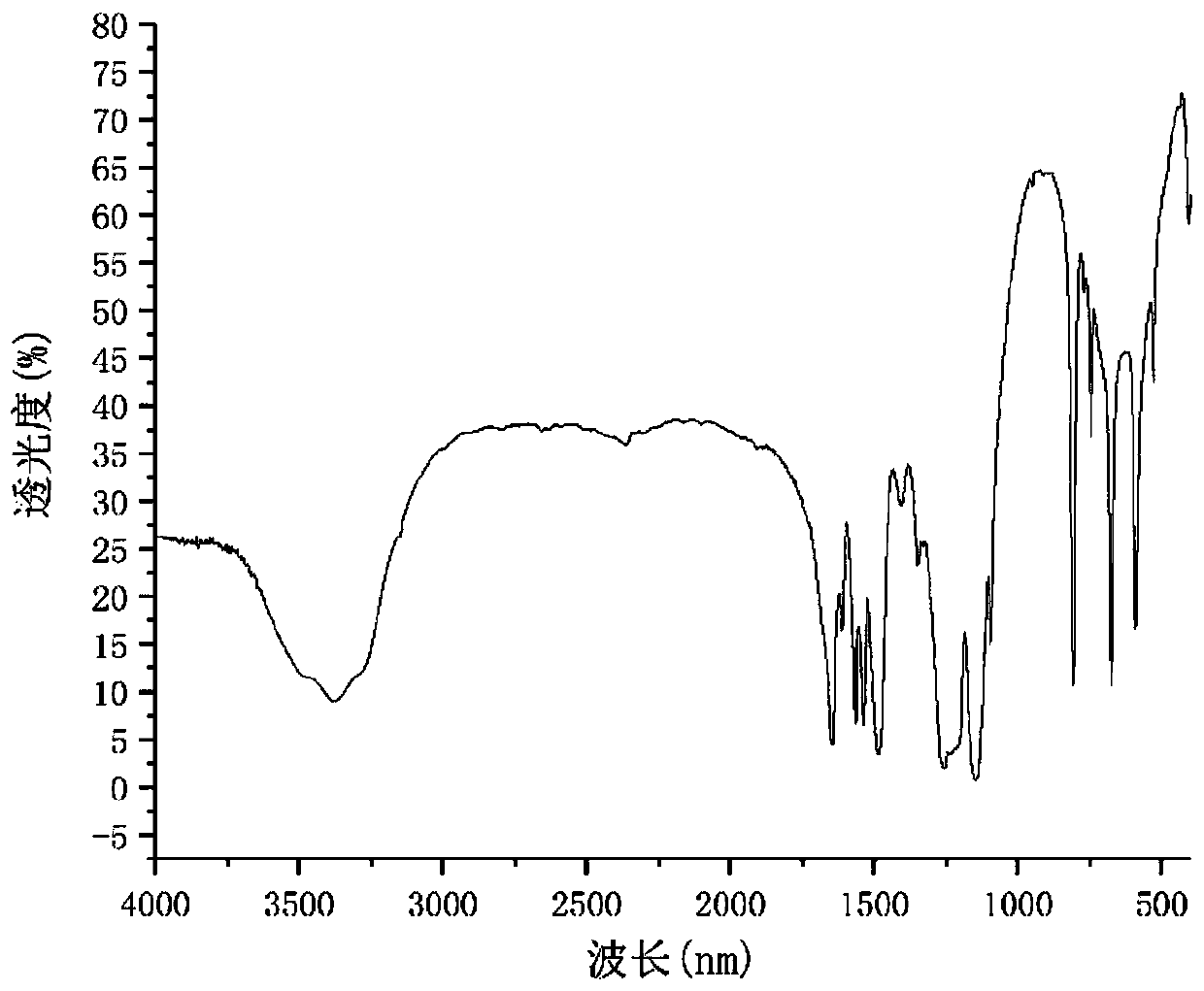
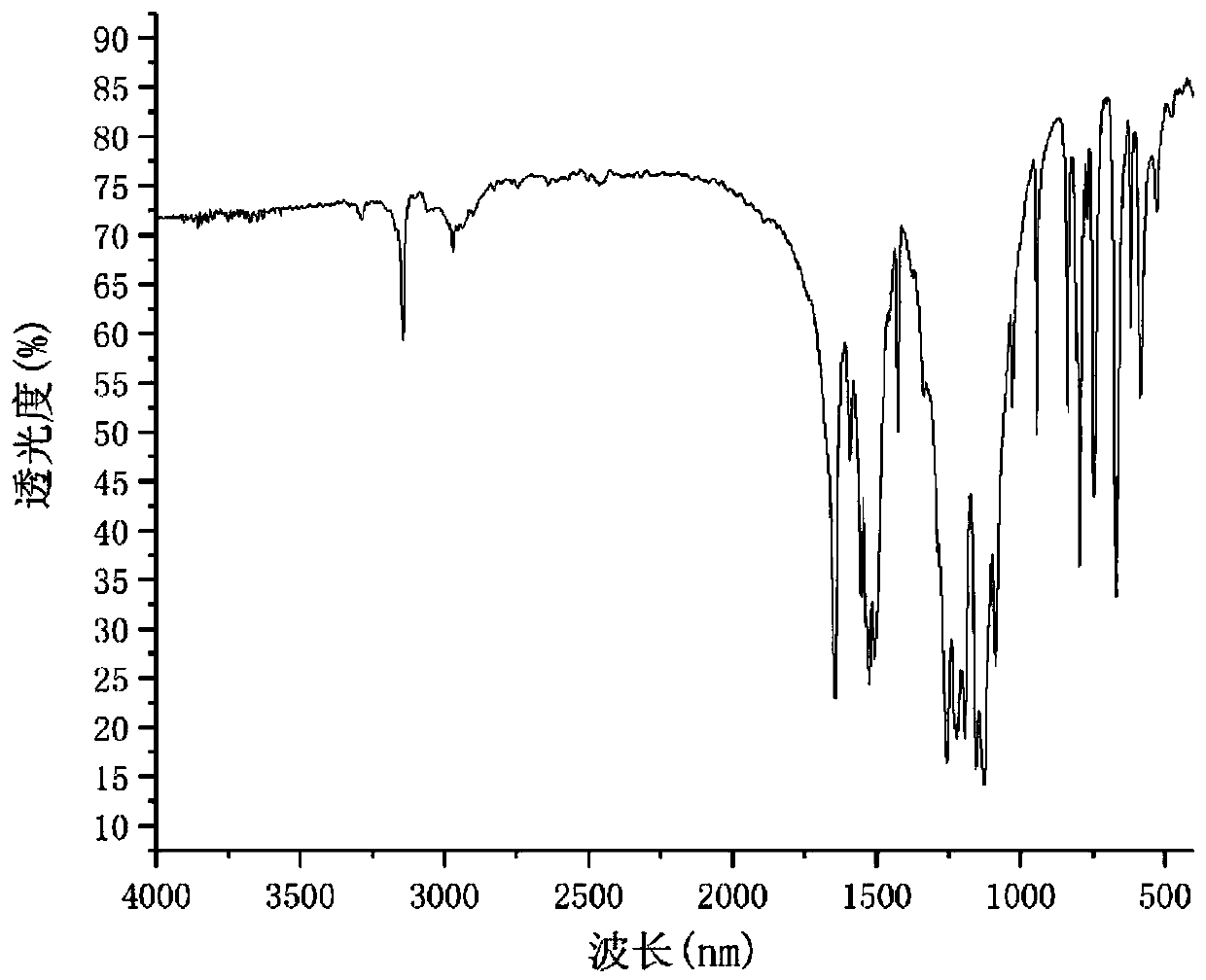
Image
Examples
Embodiment 1
[0038] The first step, the preparation of anion
[0039] Add 1.5ml of ammonia water to 30mL of absolute ethanol, then seal the flask with plastic wrap, and add 1.3ml of hexafluoroacetylacetone dropwise into the flask through a syringe at a rate of about 1mL / min, white vapor appears. After the white vapor settled, 0.8 g of cobalt chloride was added. Cobalt chloride gradually dissolved, the solution was dark red, stirred at room temperature for 5h. Ethanol was rotary evaporated at 40 °C, the crude product was redissolved in 20 mL of ethyl acetate and washed several times with 5 mL aliquots of deionized water until the aqueous fraction was loaded with AgNO 3No precipitation occurs. Ethyl acetate was evaporated by rotary evaporation at 40°C, and vacuum-dried at 40°C for 8 hours to finally obtain a red solid powder.
[0040] The second step, the preparation of cations
[0041] 20ml of N-methylimidazole and 20ml of n-bromobutane were stirred and reacted overnight at 50°C to obta...
Embodiment 2
[0046] The first step, the preparation of anion
[0047] Add 1.5ml of ammonia water to 30mL of absolute ethanol, then seal the flask with plastic wrap, and add 1.3ml of hexafluoroacetylacetone dropwise into the flask through a syringe at a rate of about 1mL / min, white vapor appears. After the white vapor settled, 0.8 g of cobalt chloride was added. Cobalt chloride gradually dissolved, the solution was dark red, stirred at room temperature for 7h. Ethanol was rotary evaporated at 50 °C, the crude product was redissolved in 20 mL of ethyl acetate and washed several times with 5 mL aliquots of deionized water until the aqueous fraction was loaded with AgNO 3 No precipitation occurs. Ethyl acetate was evaporated by rotary evaporation at 50°C, and vacuum-dried at 50°C for 9h to finally obtain a red solid powder.
[0048] The second step, the preparation of cations
[0049] 20ml of N-methylimidazole and 20ml of n-bromobutane were stirred and reacted overnight at 75°C to obtain a...
Embodiment 3
[0054] The first step, the preparation of anion
[0055] Add 1.5ml of ammonia water to 30mL of absolute ethanol, then seal the flask with plastic wrap, and add 1.3ml of hexafluoroacetylacetone dropwise into the flask through a syringe at a rate of about 1mL / min, white vapor appears. After the white vapor settled, 0.8 g of cobalt chloride was added. Cobalt chloride gradually dissolved, the solution was dark red, stirred at room temperature for 8h. Ethanol was rotary evaporated at 60 °C, the crude product was redissolved in 20 mL of ethyl acetate and washed several times with 5 mL aliquots of deionized water until the aqueous fraction was loaded with AgNO 3 No precipitation occurs. Ethyl acetate was evaporated by rotary evaporation at 60°C, and vacuum-dried at 60°C for 10 h to finally obtain a red solid powder.
[0056] The second step, the preparation of cations
[0057] 20ml of N-methylimidazole and 20ml of n-bromobutane were stirred and reacted overnight at 100°C to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com