Fusion protein and preparation method and application thereof
A fusion protein and protein technology, applied in the field of anchored fusion protein and its preparation, can solve the problems of low transfection efficiency, tumorigenicity, gene transduction failure, etc., achieve wide interaction area, sensitive reactivity, and reduce side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
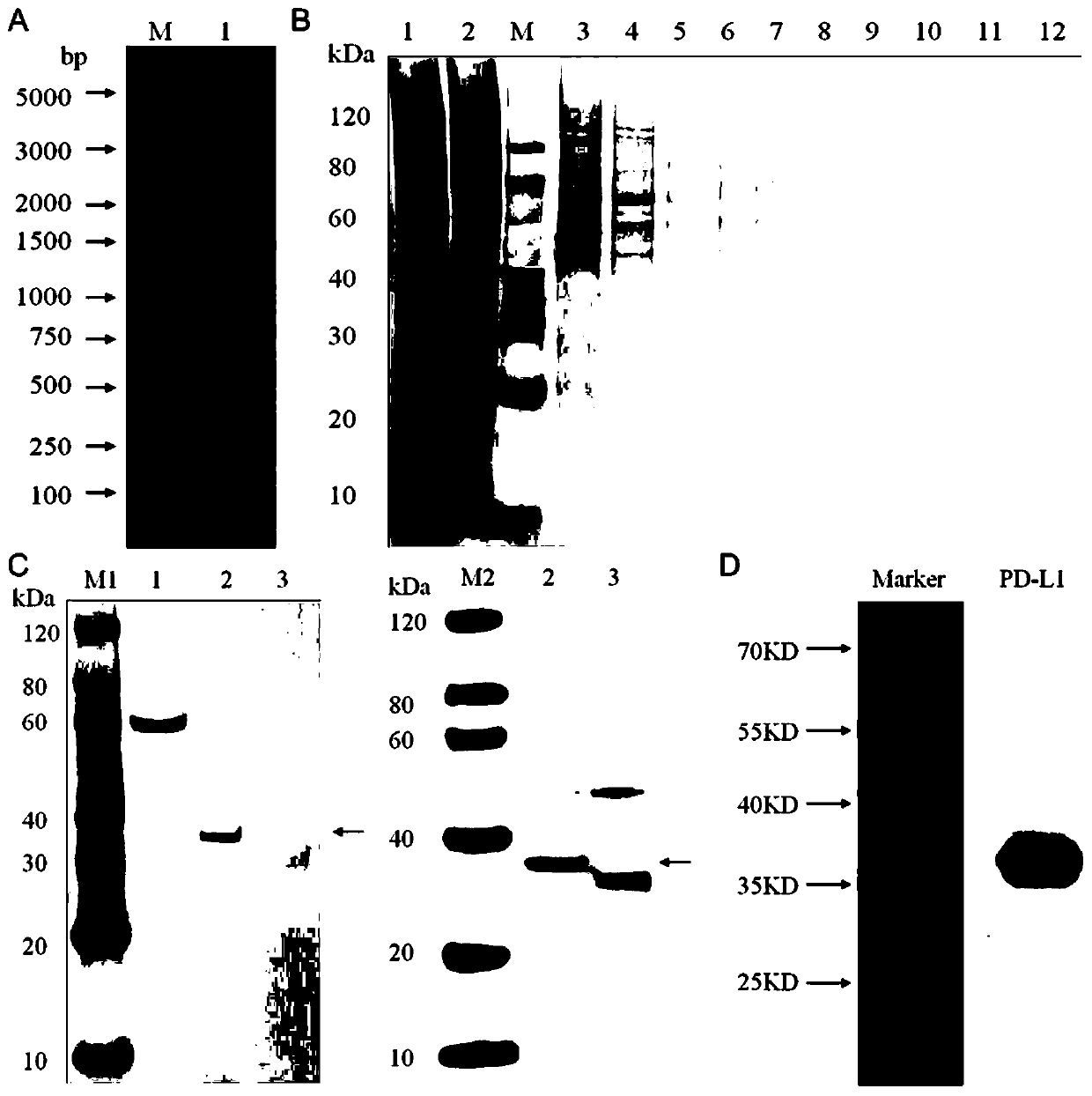
[0044] Example 1 Synthesis, purification and identification of fusion protein map-PD-L1
[0045] An embodiment of the fusion protein of the present invention, the fusion protein of this embodiment includes APT542 anchor protein and PD-L1 protein.
[0046] The preparation method of the fusion protein map-PD-L1 described in this example is: use the codon optimization software MaxCodonTM Optimization Program (V13) to optimize the protein amino acid sequence of the rat PD-L1 and the anchor protein APT542, and use the whole gene Synthesized and inserted the PD-LI+APT542 fusion gene into the pcDNA3.1(-) expression vector by double enzyme digestion, then confirmed the accuracy of the expression vector by enzyme digestion and sequencing technology, and finally transferred it to the DH5a clone strain, The transfection-grade plasmid was extracted by the plasmid extraction kit, and then the plasmid was transfected into mammalian cells HEK293 by transfection reagent for transient expressi...
Embodiment 2
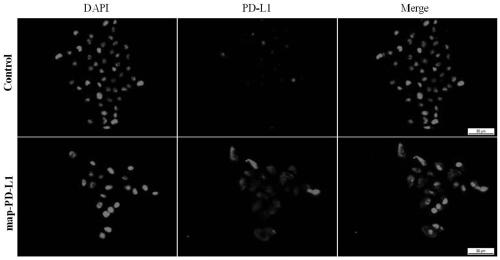
[0049] This example verifies that the fusion protein described in Example 1 can bind to renal endothelial cells cultured in vitro through in vitro experiments. The specific verification steps are as follows:
[0050] Using rat kidney endothelial cells (rgEC) cultured in vitro, the experimental group was incubated with 5ug / mL fusion anchor protein map-PD-L1 at 37 degrees for 1 hour, and the control group was treated with an equal volume of medium, and incubated The conditions and time were the same; then, the cell immunofluorescence technique was used to incubate the primary antibody anti-PD-L1, and then the secondary antibody was used for fluorescence color development to take pictures. The results of the pictures were as follows: figure 2 shown. Data analysis shows that endothelial cells can display corresponding fluorescent colors (red) after being anchored and incubated with map-PD-L1, while no obvious fluorescent colors (red) appear in the control group, indicating that ...
Embodiment 3
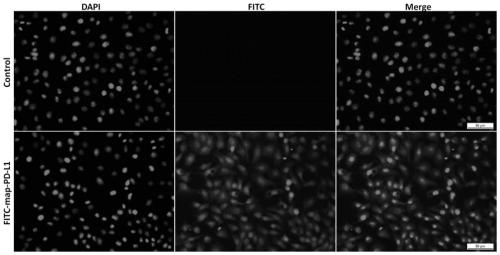
[0052] This example demonstrates through in vitro experiments that the fusion anchoring protein FITC-map-PD-L1 labeled with fluorescein also retains the ability to anchor kidney endothelial cells. The specific verification steps are as follows:
[0053] Using in vitro cultured rat kidney endothelial cells (rgEC), the experimental group was incubated with 5ug / mL concentration of FITC-labeled fusion anchor protein FITC-map-PD-L1 at 37 degrees for 1 hour, and the control group was incubated with an equal volume of The culture medium was treated with the same incubation conditions and time; then DAPI was used to stain the nuclei to observe and take pictures. The results of the pictures were as follows: image 3 shown. Data analysis shows that endothelial cells can display corresponding fluorescent colors (green) after being incubated with FITC-map-PD-L1 anchors, while no obvious fluorescent colors (green) appear in the control group, indicating fusion anchoring after fluorescein ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com