A kind of flame retardant containing polyvalent phosphorus element and preparation method thereof
A phosphorus element, multivalent technology, applied in the field of phosphorus-containing flame retardants, can solve the problems to be further improved, and achieve the effect of high flame retardant efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] ①Trephthalaldehyde reacts with 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide in a molar ratio of 1:2 under nitrogen protection in toluene solvent by heating to reflux temperature After 3 hours, the solvent was removed by rotary evaporation to give the phosphorus-containing diol.
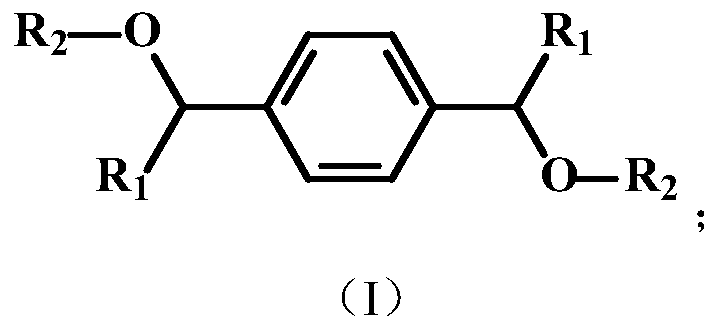
[0029] 2. The above-mentioned phosphorus-containing diol, triethylamine, and diphenyl chlorophosphate were heated to 60° C. in a toluene solvent at a reaction molar ratio of 1:2:2, reacted for 24 hours, filtered, and the solvent was removed by rotary evaporation to obtain the target Product a, whose chemical structure is shown below:
[0030]
[0031] The target product a was subjected to Fourier transform infrared spectroscopy (FT-IR), hydrogen nuclear magnetic resonance spectroscopy ( 1 H-NMR) characterization, confirmed its chemical structure as follows: FT-IR (KBr, cm -1 ): 754,934(P-O-C),1226-1201(P=O),1589(P-O-Ph). 1 H-NMR (400MHz, DMSO-d 6 , ppm): 5.40–5.13 (m, 2H, (P-CH-O) ...
Embodiment 2
[0033] ①Terephthalaldehyde and 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide, in a molar ratio of 1:2.2, were heated to reflux temperature in toluene solvent under argon protection The reaction was carried out for 6 hours, and the solvent was removed by rotary evaporation to obtain a phosphorus-containing diol.
[0034] ②The above-mentioned phosphorus-containing diol, triethylamine, and diphenylphosphinic acid chloride were heated to 100° C. in a toluene solvent at a reaction molar ratio of 1:2.2:2.2, reacted for 6 hours, filtered, and the solvent was removed by rotary evaporation to obtain the target Product b, the chemical structure of which is shown below:
[0035]
[0036] The target product b was subjected to Fourier transform infrared spectroscopy (FT-IR), hydrogen nuclear magnetic resonance spectroscopy ( 1 H-NMR) characterization, confirm its chemical structure as follows: FT-IR (KBr, cm-1): 932 (P-O-C), 1192 (P=O), 1589 (P-Ar). 1 H-NMR (400MHz, DMSO-d 6 , p...
Embodiment 3
[0038] ①Terephthalaldehyde and 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide, in a molar ratio of 1:2.1, were heated to reflux temperature in toluene solvent under the protection of helium gas The reaction was carried out for 4 hours, and the solvent was removed by rotary evaporation to obtain a phosphorus-containing diol.
[0039] ②The above phosphorus-containing diol, triethylamine and chlorodiphenylphosphine were heated to 80°C in a toluene solvent at a reaction molar ratio of 1:2.1:2.1, reacted for 12 hours, filtered, and the solvent was removed by rotary evaporation to obtain the target The product c, whose chemical structure is shown below:
[0040]
[0041] The target product c was subjected to Fourier transform infrared spectroscopy (FT-IR), hydrogen nuclear magnetic resonance spectroscopy ( 1 H-NMR) characterization, confirmed its chemical structure as follows: FT-IR (KBr, cm -1 ): 932(P-O-C), 1231(P=O), 1592cm -1 (P-Ar). 1 H-NMR (400MHz, DMSO-d 6 , ppm):...
PUM
| Property | Measurement | Unit |
|---|---|---|
| oxygen index | aaaaa | aaaaa |
| oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com