Exosome drug carrying system and applications thereof in spinal cord injury repairing
A technology for spinal cord injury and exosomes, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems such as neurotoxicity, low bioavailability, and poor biocompatibility. , to achieve the effect of promoting the recovery of behavioral function, reducing diffusion loss, and favoring differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
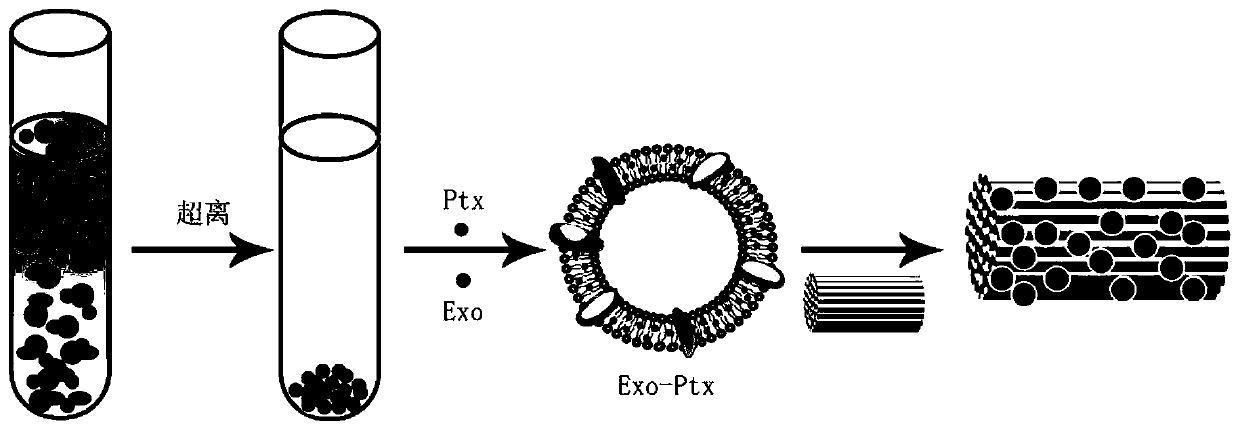
[0034] Another aspect of the embodiments of the present invention also provides the preparation method of the aforementioned exosome drug-carrying system, which includes: blending and extruding drug molecules with exosomes, so that the drug molecules are entrapped in the exosomes, Formation of exosome drug delivery system.
[0035] Further, the extruding is through an extruder with a nano-sized aperture, and the number of extruding is preferably an odd number, and then excess paclitaxel is removed by ultrafiltration.
[0036] Further, the exosomes of the mesenchymal stem cells are obtained by the following method:
[0037] The mesenchymal stem cells were cultured by conventional culture methods. At the P3 generation, the medium was replaced with serum-free exosomes, and the purified medium was collected to obtain exosomes of human umbilical cord mesenchymal stem cells.
[0038] In one embodiment of the present invention, get the P2 generation cell of logarithmic growth phase,...
Embodiment 1
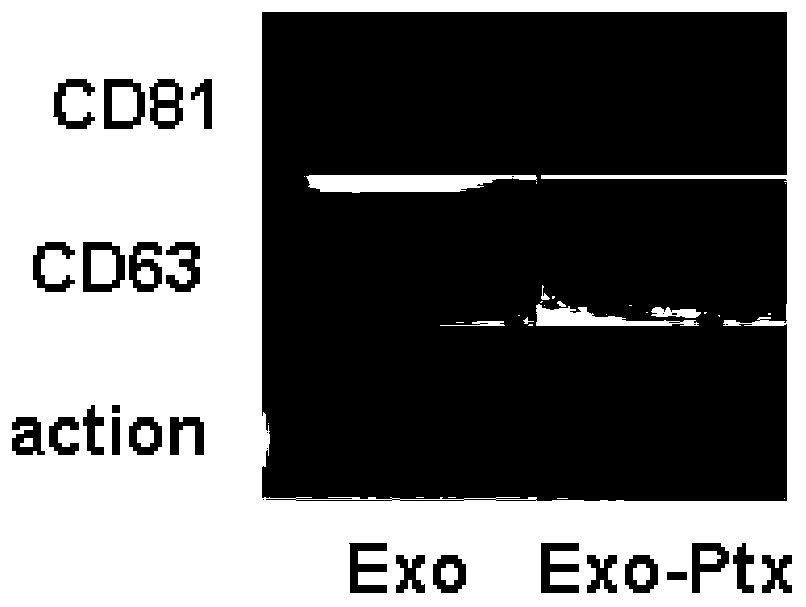
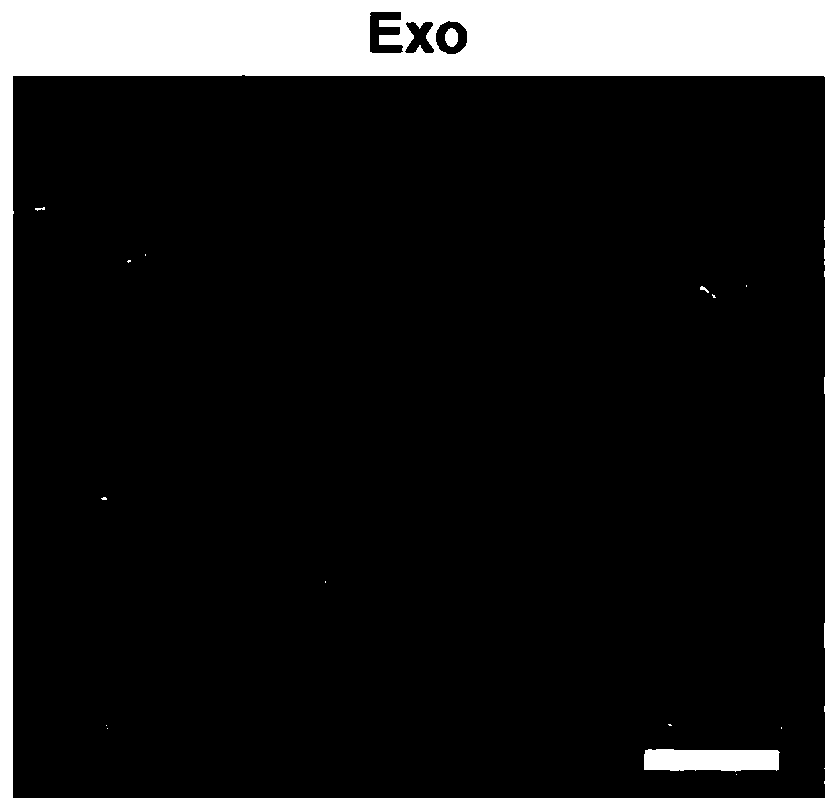
[0046] 1. Isolation and characterization of exosomes from human umbilical cord mesenchymal stem cells:
[0047] The mesenchymal stem cells were cultured by conventional culture methods, and replaced with serum-free exosome medium at the P3 generation, and the purified medium was collected to obtain human umbilical cord mesenchymal stem cell exosomes.
[0048]Take the P2 generation cells in the logarithmic growth phase and culture them at 175cm 2 In the cell culture flask, when the cell proliferation and confluence reach 70-80%, routine subculture is carried out according to the ratio of 1:4. The P3 generation cells were cultured in a medium without exosome serum, and the cell culture supernatant was collected. The cell supernatant was centrifuged at 300 g for 10 min at 4°C to remove free cells. Transfer the supernatant to another centrifuge tube and centrifuge at 2000g for 10min at 4°C to remove cell debris. Transfer the supernatant to another centrifuge tube, centrifuge at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com