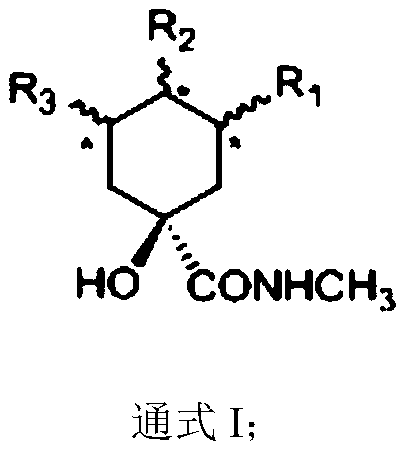
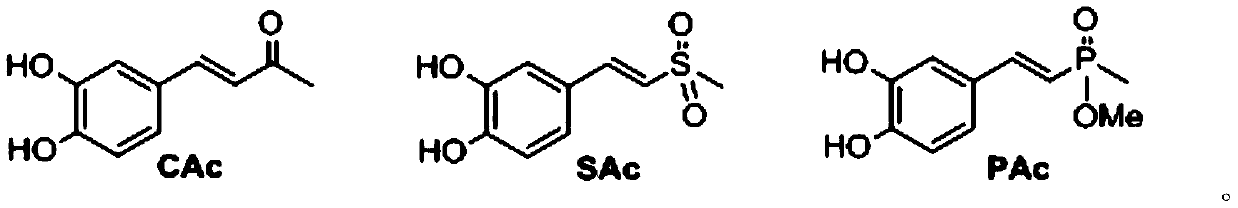
Quinic acid derivative and preparation method and application thereof
A technology of derivatives and quinic acid, applied in the preparation of sulfonamides, carboxylic acid amides, organic compounds, etc., can solve problems such as easy hydrolysis, short half-life, and unstable structure of dicaffeoyl compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
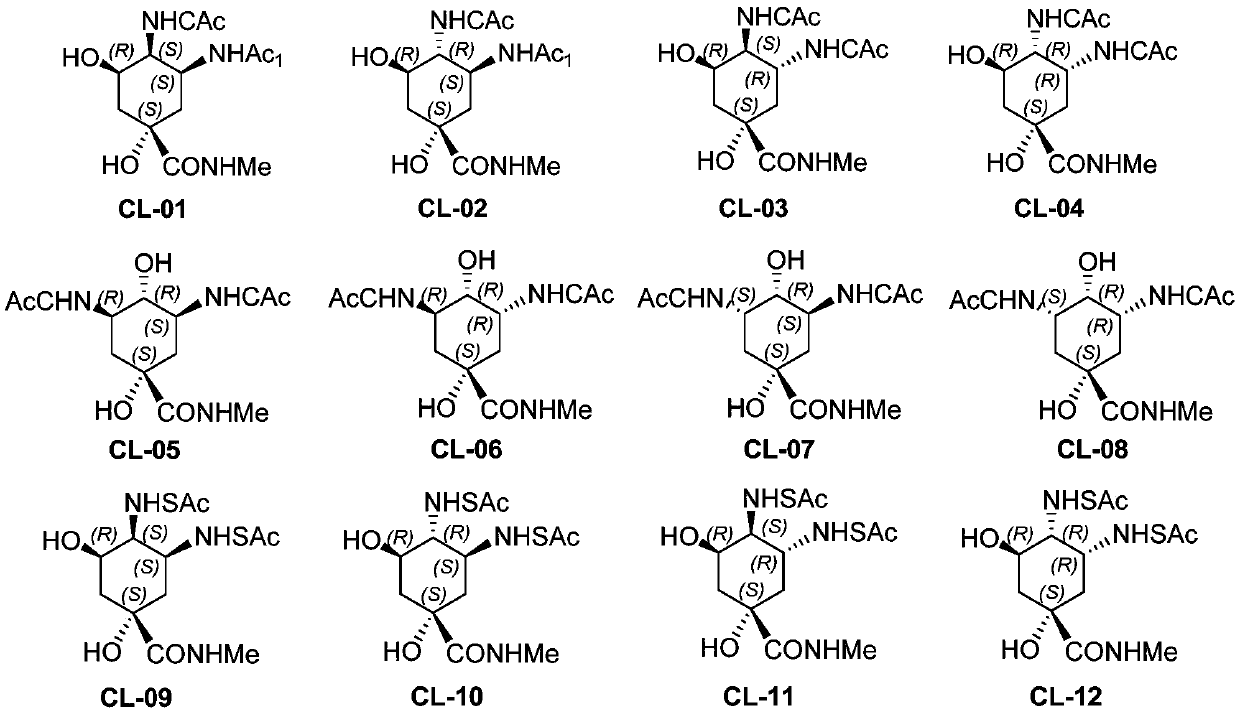
[0138] Example 1 Preparation of N-methyl-N', N'-dicaffeoyl-3,4-diamino-1,5-dihydroxycyclohexane-1-carboxamide (CL-01~CL-04) and separation and purification
[0139] (1) Preparation of (E)-3-(benzo[d][1,3]dioxol-5-yl)acrylic acid
[0140] (1a) Preparation of benzo[d][1,3]dioxolene (pipercycline)
[0141] Weigh catechol (110.1 mg, 1.0 mmol) into a 50 mL round bottom flask, add 15 mL of N,N-dimethylformamide, stir to dissolve the catechol, then add diiodomethane (321.4 mg, 1.2mmol) and sodium bicarbonate (210.0mg, 2.5mmol), heated at 80°C for 12h. After the reaction was completed, the product was slowly poured into ice water while stirring, extracted 3 times with ethyl acetate, the organic layer was dried over anhydrous sodium sulfate, ethyl acetate was removed by rotary evaporation under reduced pressure, and silica gel column chromatography (Beijing Shinwell Glass Instrument Co., Ltd. Co., Ltd.) separation and purification (petroleum ether: ethyl acetate = 10:1, V:V) to obta...
Embodiment 2
[0171]Example 2 Preparation of N-methyl-N', N'-dicaffeoyl-3,5-diamino-1,4-dihydroxycyclohexane-1-carboxamide (CL-05~CL-08) and separation and purification
[0172] (1) The preparation of (E)-3-(benzo[d][1,3]dioxopenten-5-yl)acrylic acid was carried out according to the step (1) in Example 1.
[0173] (2) Preparation of N-methyl-3,5-diamino-1,4-dibenzyloxycyclohexane-1-carboxamide
[0174] (2a) The preparation of methyl quinic acid was carried out according to (2a) in the step (2) of Example 1.
[0175] (2b) The preparation of N-methylquinic amide was carried out according to (2b) in step (2) of Example 1.
[0176] Preparation of (2c')N-methyl-3,5-bis(tert-butyldimethylsilyloxy)quininamide
[0177] Weigh the N-methylquinine amide (1.74g, 8.5mmol) that step (2b) obtains in the dry 100mL round-bottomed flask, dissolve with an appropriate amount of dry THF, then add imidazole (3.50mg, 51.0 mmol), and finally a solution of tert-butyldimethylsilyl chloride (TBSCl) (2.33 g, 20.5 ...
Embodiment 3
[0196] Example 3 N-methyl-N', N'-dicaffeyl-3,4-diamino-1,5-dihydroxycyclohexane-1-carboxamide (CL-09~CL-12) Preparation and separation and purification
[0197] (1a-b) The preparation of (E)-2-(benzo[d][1,3]dioxopenten-5-yl)ethylene-1-sulfonic acid according to the procedure in Example 1 step (1) (1a) and (1b) yield piperonal.
[0198] Preparation of (1c')(E)-2-(benzo[d][1,3]dioxol-5-yl)ethylene-1-sulfonic acid
[0199] In a 250 mL round bottom flask, piperonal (12 g, 79.9 mmol) and methanesulfonic anhydride (20.9 g, 120 mmol) obtained in step (1a-b) were dissolved in 150 mL of dry THF, and NaH (2.9 g, 120 mmol) was added in portions , and then the reaction solution was moved to an oil bath at 115° C. and heated to reflux for about 2 hours. The reaction process was monitored by TLC until the complete disappearance of piperonal, indicating that the reaction was complete. After the reaction solution was cooled to room temperature, the reaction solution was slowly poured into...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com