A kind of fluorescent probe and preparation method thereof for differentiating and detecting cys/hcy and gsh
A technology of fluorescent probe and synthesis method, applied in the field of fluorescent probe, can solve the problem of different detection of thiols rarely reported, and achieve the effects of simple synthesis method, obvious detection signal and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation and characterization of NBD-O-1
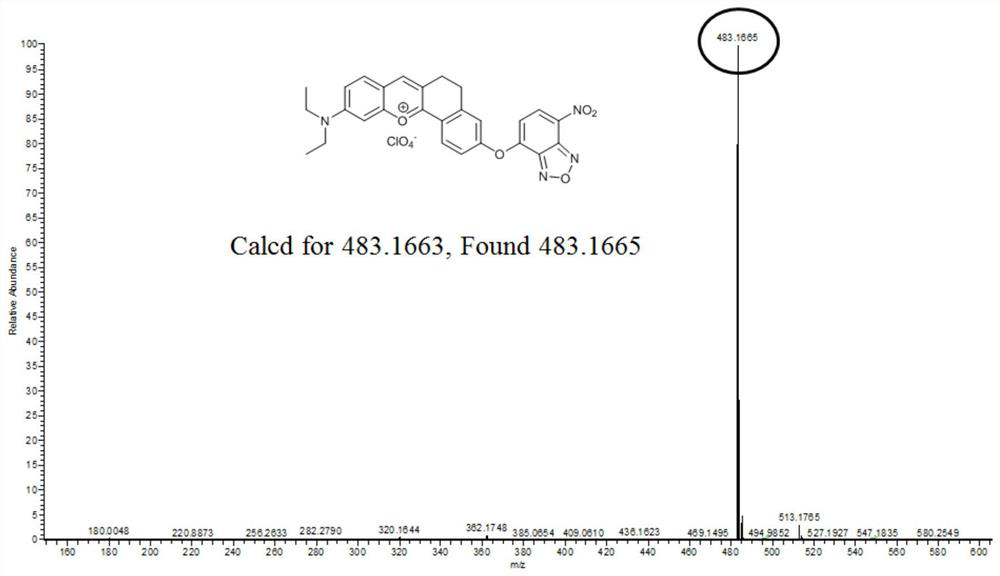
[0033] (1) Preparation of Compound 1: In a 100 ml round bottom flask, 4-diethyl amino hydrarse (1.93 g, 10 mmol), 6-hydroxy-1-tetrahemethoelene (1.62 g, 10 mmol) and high Hichlorous acid (3 mL) is dissolved in 20 ml of acetic acid and the mixture was refluxed for 1.5 hours. After cooling to room temperature, the solution was poured into a mixture of ethyl acetate (15 mL) and petroleum ether (15 mL). The precipitate was filtered and washed with ethanol, then dried in vacuo to give a pure deep purple solid compound 1 (2.88 g, yield: 90%). 1 HNMR (600MHz, DMSO-D 6 Δ11.11 (S, 1H), 8.63 (S, 1H), 8.16 (D, J = 8.6 Hz, 1H), 7.91 (D, J = 9.3 Hz, 1H), 7.41 (D, J = 9.3 Hz, 1H), 7.27 (S, 1H), 6.95 (D, J = 8.6Hz, 1H), 6.87 (S, 1H), 3.67 (Q, J = 6.9 Hz, 4H), 3.01 (S, 4H), 1.24 ( T, J = 7.0 Hz, 6H). 13 C NMR (150MHz, DMSO-D 6 ) δ164.73, 164.31, 158.24, 155.23, 148.36, 146.21, 132.03, 129.40, 12.70, 117.99, 117.67, 117.66, 116.16, 96.98, 25.11,...
Embodiment 2
[0036] (1) The fluorescent probe stock solution of 2 mM NBD-O-1 was prepared with dimethyl sulfoxide (DMSO); 2 mm Cys / Hcy and GSH solution were formulated with distilled water.
[0037] (2) 2ML CH 3 CN / PBS buffer (V / V = 2 / 8, pH = 7.4) and 10 μl of fluorescent probe stock solution Add a fluorescence composite vendor to measure the fluorescence spectrum of the probe on a fluorescent spectrophotometer, and then gradually add different volumes. CYS / HCY and GSH solution, the fluorescence spectrum was measured on a fluorescence spectrophotometer, and two new fluorescence emission peaks were added at 550 nm and 625 nm. As Cys / Hcy added fluorescence intensity gradually increased. Until the basics do not change ( Figure 4 (A), (b)); after the addition of GSH, the probe only appears at 625 nm, and the new fluorescence emission peak ( Figure 4 In (c)), distinguishing detection can be realized.
[0038] (3) For Cys, the fluorescence intensity at the Cys concentration of 550 nm is ...
Embodiment 3
[0040] In different fluorescent polyvisses, 2 ml of CH is added separately 3 CN / PBS buffer (V / V = 2 / 8, pH = 7.4) and 10 μl of fluorescent probe reserve, and Cys / Hcy and GSH were added to other amino acids at 50 μm and 20 μm, and 10 equivalents. Aqueous solution, including ALA, ASP, ASN, ARG, GLY, GLU, GLN, HIS, IIE, LEU, LYS, MET, PHE, PRO, SER, TYR, THR, TRP AND VAL, so that its final concentration is 100 μm, then Fluorescence spectroscopy on fluorescence spectrophotometer, see the measurement results Image 6 . Experiments have shown that these amino acids do not interfere with the detection of CYS / Hcy and GSH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com