A kind of spiropyran-naphthalimide derivative and its synthesis method and application
A technology of naphthalimide and synthesis method, applied in the field of spiropyran derivatives, can solve problems such as unexplained mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation and characterization of NSPs
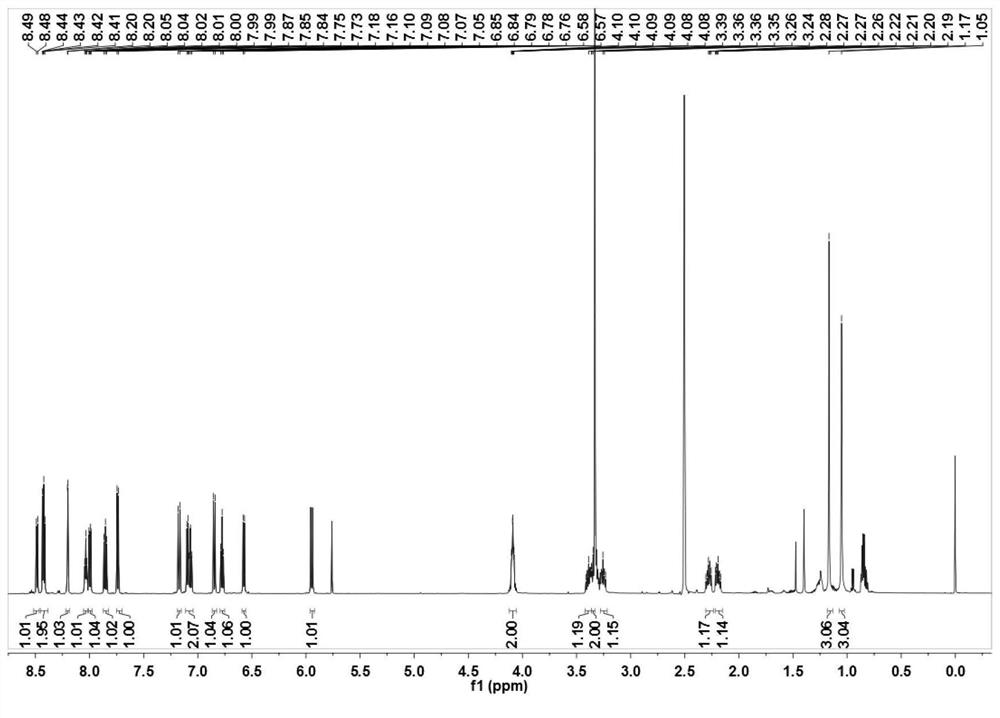
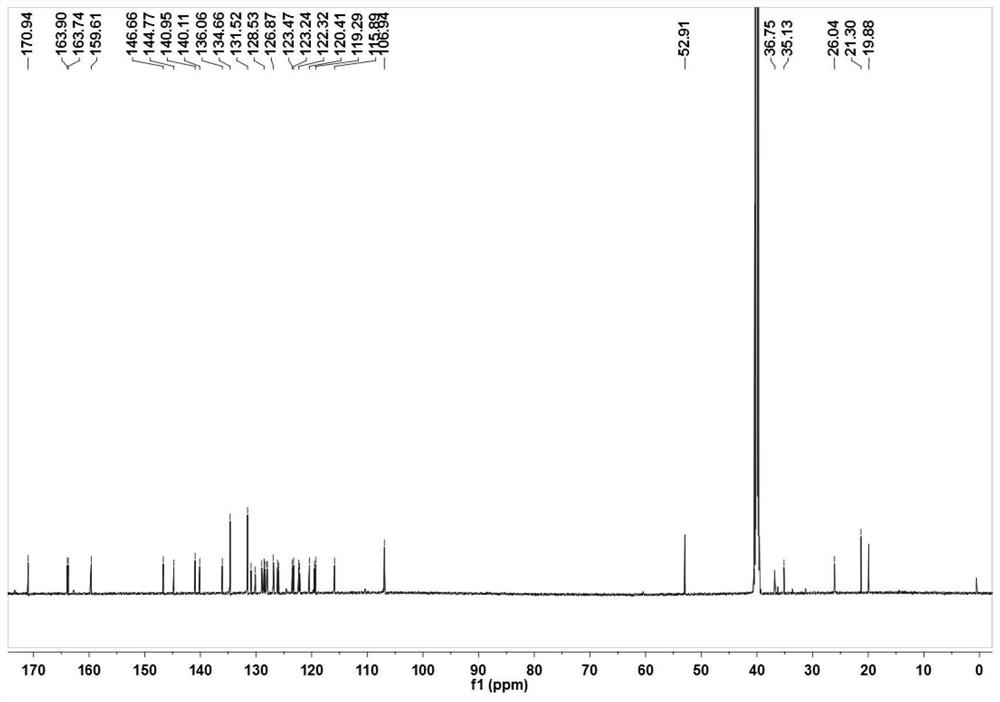
[0053] Mix 2,3,3-trimethyl-3H-indole (3.18g, 20mmol) and 3-bromopropionic acid (6.08g, 40mmol) in 30mL of anhydrous acetonitrile, and heat the mixture at 85°C for 12 hours After the reaction was completed, the solvent was removed under reduced pressure, and the residue obtained was washed three times with 20 mL of ether to remove unreacted raw materials, and the residue was recrystallized with dichloromethane (5 mL) and acetone (25 mL) by volume to obtain a light purple powder product (3.78 g, produced rate: 57.7%). 1 H NMR(600MHz,DMSO)δ12.69(s,1H),7.99(d,J=4.3Hz,1H),7.85(d,J=4.0Hz,1H),7.65–7.59(m,2H),4.66 (t,J=6.8Hz,2H),2.99(t,J=6.9Hz,2H),2.86(s,3H),1.53(s,6H). 13 C NMR (151MHz, DMSO) δ198.40(s), 172.01(s), 142.23(s), 141.32(s), 129.82(s), 129.39(s), 123.95(s), 116.04(s), 54.73 (s), 43.99(s), 31.57(s), 22.34(s), 14.82(s).
[0054] 1-(2-carboxyethyl)-2,3,3-trimethyl-3H-indole-1-bromide (3.27g, 10mmol), 5-nitrosalicylaldehyd...
Embodiment 2
[0059] Prepare PBS buffer solution with pH=7.4 and concentration of 10mM, prepare 2mM NSP in DMSO solution, prepare 2mM hydrogen sulfide aqueous solution; take 2mL C 2 h 5 Add OH / PBS (v / v=1:1, pH=7.4) solution and 10 μL NSP DMSO solution to a fluorescent cuvette, take the aqueous solution of hydrogen sulfide, and gradually add it to the cuvette with a micro-injector In the process, it was detected on a spectrophotometer while adding the sample. With the addition of hydrogen sulfide, the fluorescence intensity at 540nm gradually increased. Fluorescence emission map see Figure 4 .
Embodiment 3
[0061] Prepare PBS buffer solution with pH=7.4 and concentration of 10mM, prepare 2mM NSP in DMSO solution, prepare 2mM hydrogen sulfide aqueous solution; add 2mL of C 2 h 5 OH / PBS (v / v=1:1, pH=7.4) solution and 10 μL NSP in DMSO solution, then add 10 times the equivalent of other analytes and hydrogen sulfide: NSP, Cys, Hcy, GSH, SO 4 2- ,SCN - ,S 2 o 3 2- ,S 2 o 5 2- ,AcO - ,CO 3 2- ,NO 3 - , SO 3 2- ,S 2- , the aqueous solution is detected on a fluorescence spectrophotometer, and the fluorescence intensity histogram at 540nm corresponding to different analytes is drawn (see Figure 5 ). Hydrogen sulfide significantly increases the fluorescence intensity of the detection system at 540 nm, and other analytes basically do not cause changes in the fluorescence intensity of the detection system.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com