Lithium secondary battery additive, preparation method thereof, and application thereof
A lithium secondary battery and additive technology, which is applied in secondary batteries, circuits, electrical components, etc., can solve the problems of air-stable solid electrolyte materials, etc., and achieve improved electrode ion rapid transmission, high ion conductivity, and preparation methods simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 glass-ceramic phase Li 3 Y 1-d In d Cl 6 Additives and their preparation
[0045] Put 30 mmoles of LiCl (1.29 g), 10-10a mmoles of InCl 3 and 10a mmol of YCl 3 After grinding, place it in a zirconia ball mill jar with a ball-to-material ratio of 30:1, and then seal the ball mill for 30 hours at a milling speed of 550 rpm. The sample obtained after ball milling is the glass-ceramic phase Li 3 Y 1-d In d Cl 6 additive. where d is 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 and 1.0.
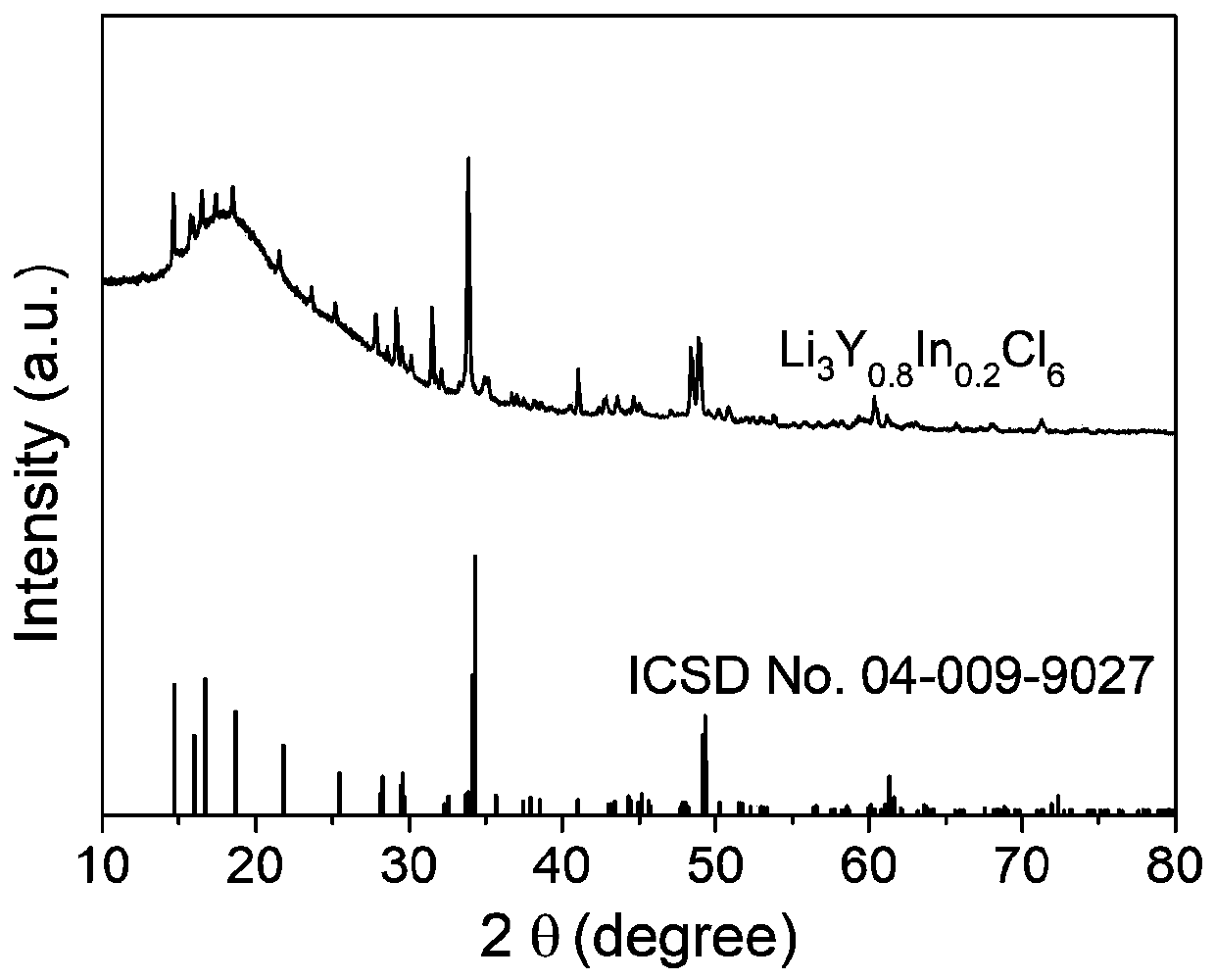
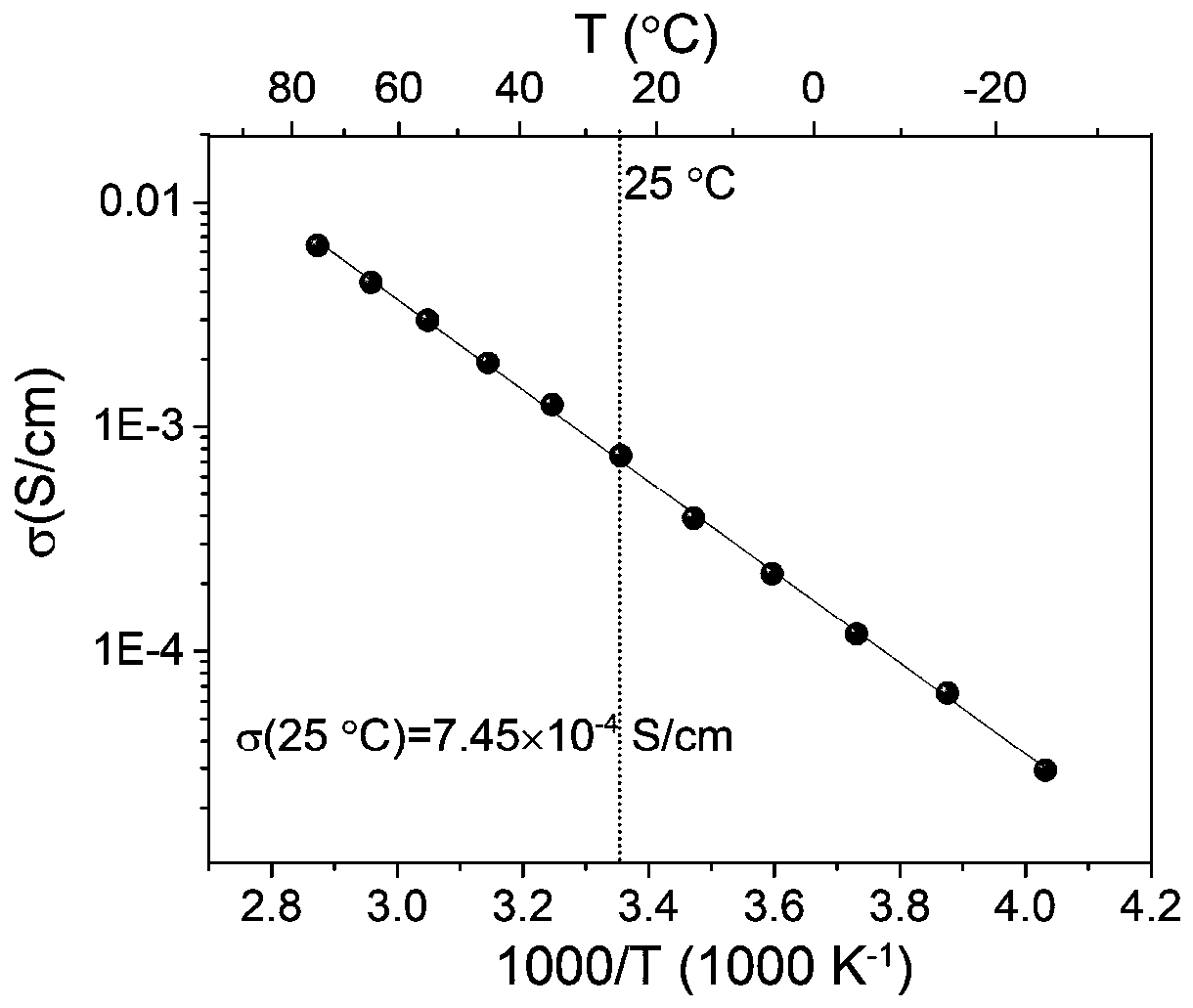
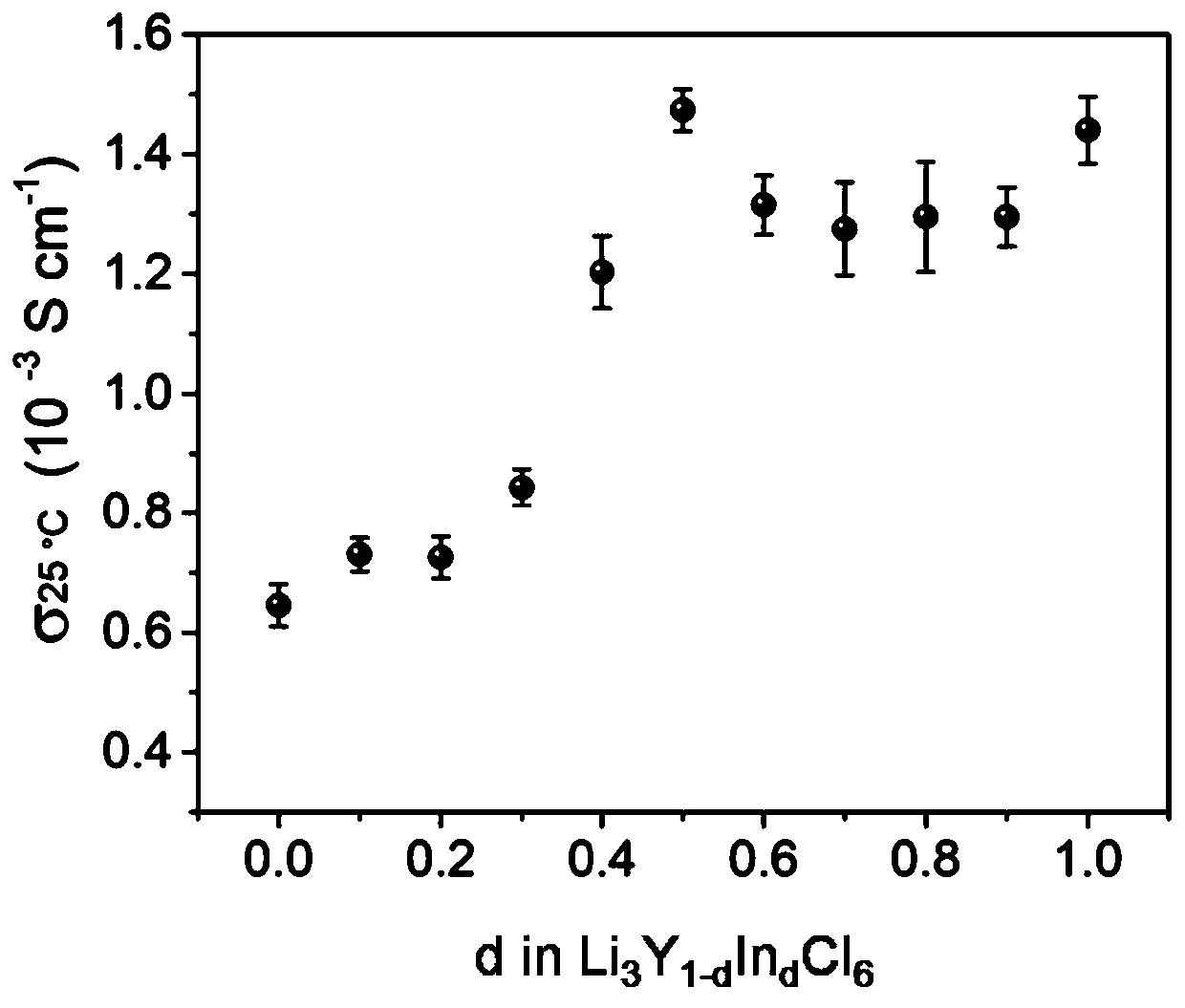
[0046] figure 1 , figure 2 The glass-ceramic phase Li that present embodiment makes respectively 3 Y 1-d In d Cl 6 (d=0.2) X-ray diffraction pattern, variable temperature ion conductivity pattern. image 3 It is the above-mentioned value of d and the change curve of the ion conductance of the corresponding product.
Embodiment 2
[0047] Embodiment 2 crystalline phase Li 3 InCl 6 Additives and their preparation
[0048] Put 30 mmol of LiCl (1.29 g), 10 mmol of InCl 3 (2.21 g) was ground and placed in a zirconia ball mill jar with a ball-to-material ratio of 20:1, followed by sealed ball milling for 20 hours at a ball milling speed of 550 rpm. The intermediate product obtained after ball milling was reacted at 450° C. for 10 hours in a sealed quartz tube. The resulting product is the crystalline phase Li 3 InCl 6 additive.
[0049] Figure 4 , Figure 5 The crystalline phase Li that this embodiment makes respectively 3 InCl 6 The X-ray diffraction pattern and the temperature-varying ion conductivity pattern.
Embodiment 3
[0050] Embodiment 3 glass phase Li 3 NbCl 8 Additives and their preparation
[0051] The preparation method is similar to Example 1, except that the raw materials used are as follows: 30 millimoles of LiCl (1.29 grams) and 2.7 grams of NbCl 5 ; The ball milling speed was changed to 450 revolutions per minute, and the ball milling time was 10 hours. Glass phase Li can be obtained after the precursor is ball milled 3 NbCl 8 additive.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com