A boron-induced amorphous layered double hydroxide electrocatalyst and its preparation and application
A kind of hydroxide electrocatalyst technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
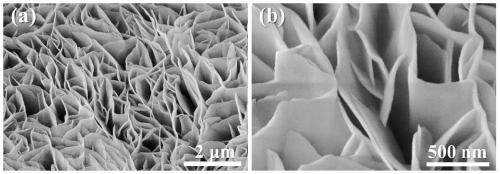
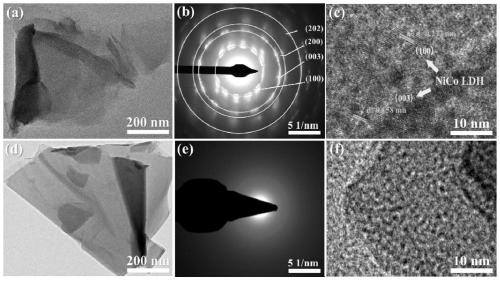
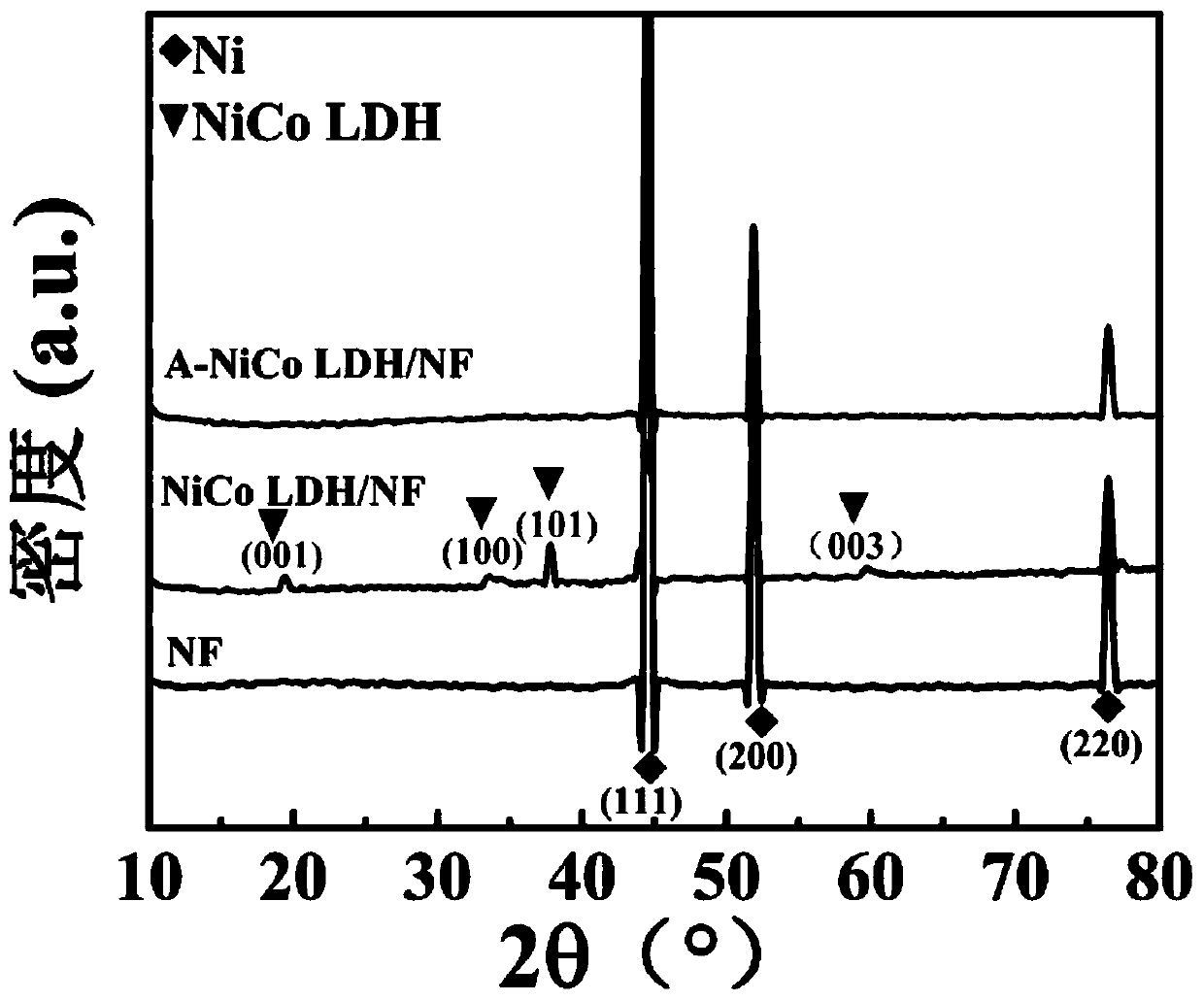
[0033] The preparation of boron-induced amorphous nickel-cobalt layered double hydroxide nanosheet electrocatalyst (3D A-NiCo LDH / NF) involves the following steps:
[0034] (1) The in-situ growth method is used to generate crystalline nickel-cobalt layered double hydroxide nanosheet precursor (3D NiCo LDH / NF) on the blank nickel foam with three-dimensional structure: intercept the blank nickel foam for pretreatment, including sequentially using Deionized water, acetone, and ethanol were ultrasonicated for 15 minutes, and then the blank nickel foam was rinsed with deionized water and dried at room temperature; then 8.8g of Ni(NO 3 ) 2 ·6H 2 O, 4.4g of Co(NO 3 ) 2 ·6H 2 O. The urotropine of 5g is dissolved in the mixed solvent of 50mL deionized water and 150mL ethanol, transfers in the beaker after ultrasonic 30min. Then put the pretreated blank nickel foam into the above solution, make it completely submerged, place it in an oven, react at 90°C for 9 hours, and after cooli...
Embodiment 2
[0044] The preparation of boron-induced amorphous nickel layered double hydroxide nanosheet electrocatalyst (3D A-Ni LDH / NF) involves the following steps:
[0045] (1) The precursor of crystalline nickel layered double hydroxide nanosheets (3D Ni LDH / NF) was grown on the blank nickel foam with three-dimensional structure by in-situ growth method: the blank nickel foam was intercepted for pretreatment, including sequentially using Deionized water, acetone, and ethanol were ultrasonicated for 15 minutes, and then the blank nickel foam was rinsed with deionized water and dried at room temperature; then 13.2g of Ni(NO 3 ) 2 ·6H 2 O. The urotropine of 5g is dissolved in the mixed solvent of 50mL deionized water and 150mL ethanol, transfers in the beaker after ultrasonic 30min. Then put the pretreated blank nickel foam into the above solution, make it completely submerged, place it in an oven, react at 90°C for 9 hours, and after cooling at room temperature, take out the foam nick...
Embodiment 3
[0049] The preparation of boron-induced amorphous cobalt layered double hydroxide nanosheet electrocatalyst (3D A-Co LDH / NF) involves the following steps:
[0050] (1) The crystalline cobalt layered double hydroxide nanosheet precursor (3D Co LDH / NF) was grown on the blank nickel foam with three-dimensional structure by in-situ growth method: the blank nickel foam was intercepted for pretreatment, including sequentially using Deionized water, acetone, and ethanol were ultrasonicated for 15 minutes, and then the blank nickel foam was rinsed with deionized water and dried at room temperature; then 10-15g of Co(NO 3 ) 2 ·6H 2 O. The urotropine of 3-8g is dissolved in the mixed solvent of 50mL deionized water and 150mL ethanol, transfers in the beaker after ultrasonic 30min. Then put the pretreated blank nickel foam into the above solution, make it completely submerged, place it in an oven, react at 90°C for 9 hours, and after cooling at room temperature, take out the foam nicke...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com