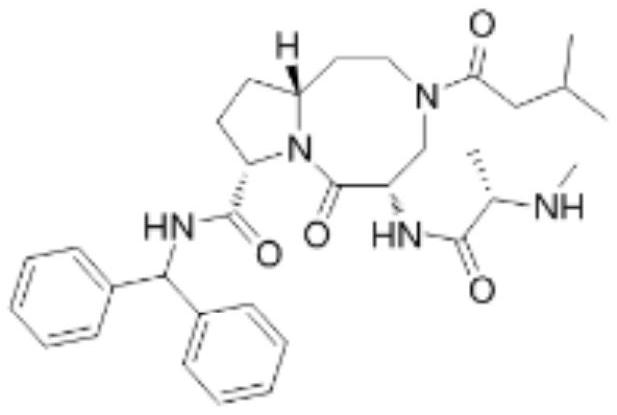
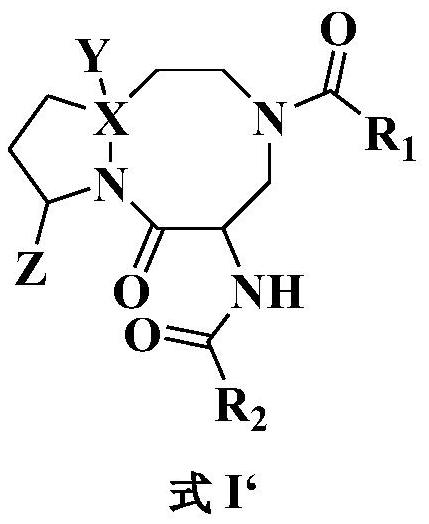
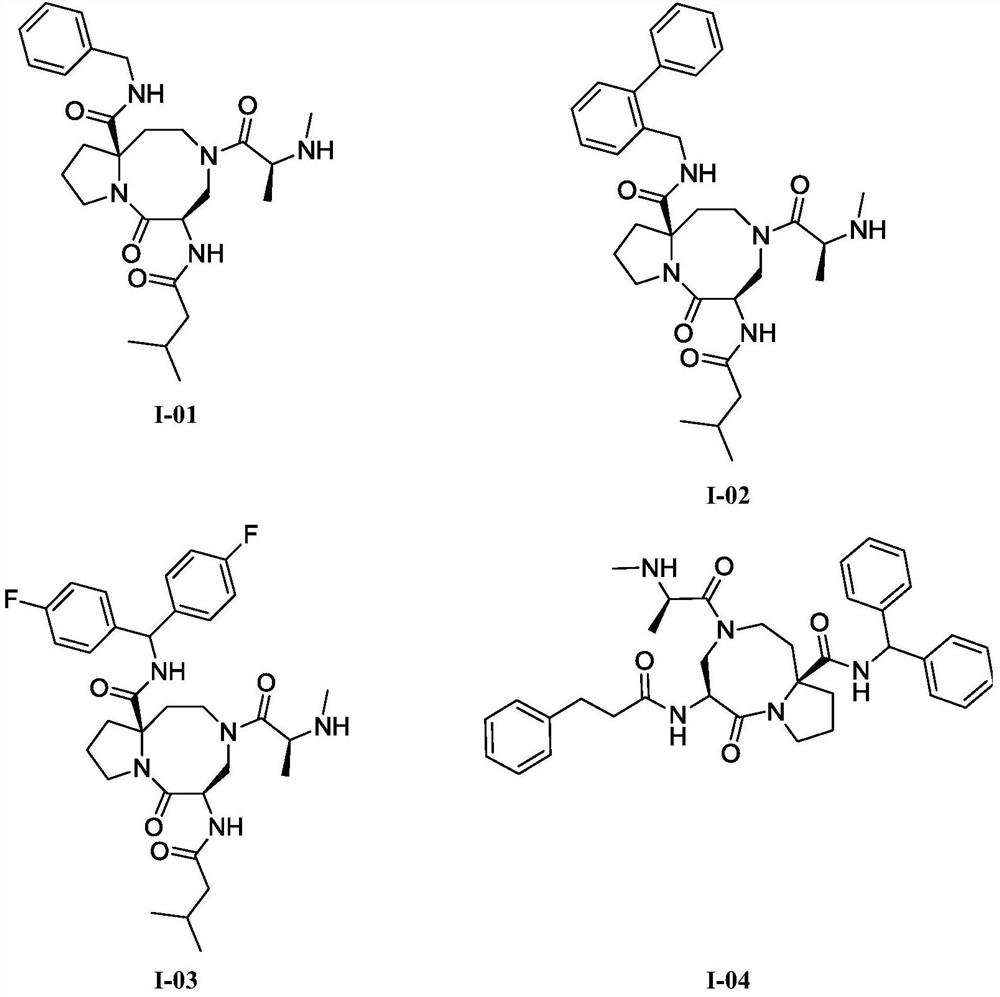
An anti-tumor diazobicyclic apoptosis protein inhibitor
A technology of cycloalkylamine and compound, applied in the field of medicinal chemistry, can solve the problems of weak effect on solid tumors, and achieve the effects of good binding affinity, good IAP inhibitory activity, and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1: Preparation of methyl pyrrolidine-2-carboxylate
[0094]
[0095] Take 40 g (0.35 mol) of proline and 250 mL of methanol in a 500 mL single-neck reaction flask, cool to 0°C, and take another 45 g (0.38 mol) of thionyl chloride in a 250 mL constant pressure dropping funnel, slowly add dropwise to the above In the solution system, heat was released with the dropwise addition system, and the internal temperature was controlled to be less than 5°C, and the dropwise addition was completed in about 20 minutes. The temperature was raised to 70°C to obtain a clear solution, which was refluxed for 3 hours. After cooling to room temperature, the solvent was evaporated under reduced pressure to obtain 43 g of a light yellow oily liquid, and the crude product was directly used in the next reaction.
Embodiment 2
[0096] Example 2: Preparation of methyl N-Boc pyrrolidine-2-carboxylate
[0097]
[0098] Take 43g (0.33mol) methyl pyrrolidine-2-carboxylate and 400mL dichloromethane in a 2L single-neck reaction flask, add 88g (0.87mol) triethylamine to obtain a white emulsion, stir at room temperature for 15 minutes, and cool to 0°C.
[0099] Another 152 g (0.69 mol) of Boc acid anhydride was dissolved in 600 mL of dichloromethane in a constant pressure funnel, and added dropwise to the above system, a large number of bubbles were released with the dropwise addition, exothermic, and the drop rate was controlled to maintain the system temperature <10 ° C. After the dropwise addition, a white suspension was obtained, which was stirred at room temperature for 12 hours. Filtration to remove the white precipitate, the filtrate was evaporated under reduced pressure to obtain a white suspension, which was washed with 300 mL of citric acid solution (0.5M), extracted with ether (3*500 mL), the o...
Embodiment 3
[0100] Example 3: Preparation of N-Boc-2-propenyl-pyrrolidine-2-carboxylic acid methyl ester
[0101]
[0102] Take 20 g (87.3 mmol) of methyl N-Boc pyrrolidine-2-carboxylate and 200 mL of tetrahydrofuran in a 500 mL three-neck flask, under nitrogen protection, and cool to -78°C.
[0103] Another 105mL (105mmol, 1M) of lithium bistrimethylsilylamide was taken in a constant pressure dropping funnel, added dropwise to the above system, the drop rate was controlled, and the temperature in the system was maintained <-78°C to obtain a yellowish brown solution. After the dropwise addition was completed, the mixture was stirred at -78°C for 1.5 hours.
[0104] Separately, 15.9 g (131 mmol) of allyl bromide was dissolved in 100 mL of tetrahydrofuran, and added dropwise to the above system. Return to room temperature and stir for 1 hour to obtain a reddish-brown solution. TLC detected that the reaction was complete, and 50 mL of water was added to quench the reaction. Extraction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com