Medical phospholipid compound and medical composition and application thereof
A technology of phospholipid compounds and compositions, applied in the field of medicine, can solve problems such as drug resistance, achieve strong drug efficacy, improve efficiency, and excellent anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
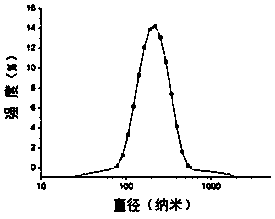
Image
Examples
Embodiment 1
[0087] Stearic acid (or palmitic acid, lauric acid)-sn-2-mercaptopropionic acid (or undecanoic acid) glycerophosphocholine, stearic acid (or palmitic acid, lauric acid)-sn-2-hydroxyethyldi Synthesis of Thiopropionic Acid (or Undecanoic Acid) Glycerophosphocholine
[0088] The general synthesis procedure is as follows: an appropriate amount of trityl (trt) protected mercaptopropionic acid (or undecanoic acid), stearic acid (or lauric acid, palmitic acid,) lysophospholipids is dissolved in dimethylsulfoxide, added Catalytic amounts of DCC and DMAP were stirred at room temperature for 12 hours. After the reaction was completed, it was precipitated with ether, washed with distilled water, extracted with dichloromethane, and the organic phase was dried with magnesium sulfate. After concentration, it is separated by column chromatography to obtain stearic acid (or palmitic acid, lauric acid)-sn-2-trt-mercaptopropionic acid (or undecanoic acid) glycerophosphocholine. Deprotection w...
Embodiment 2
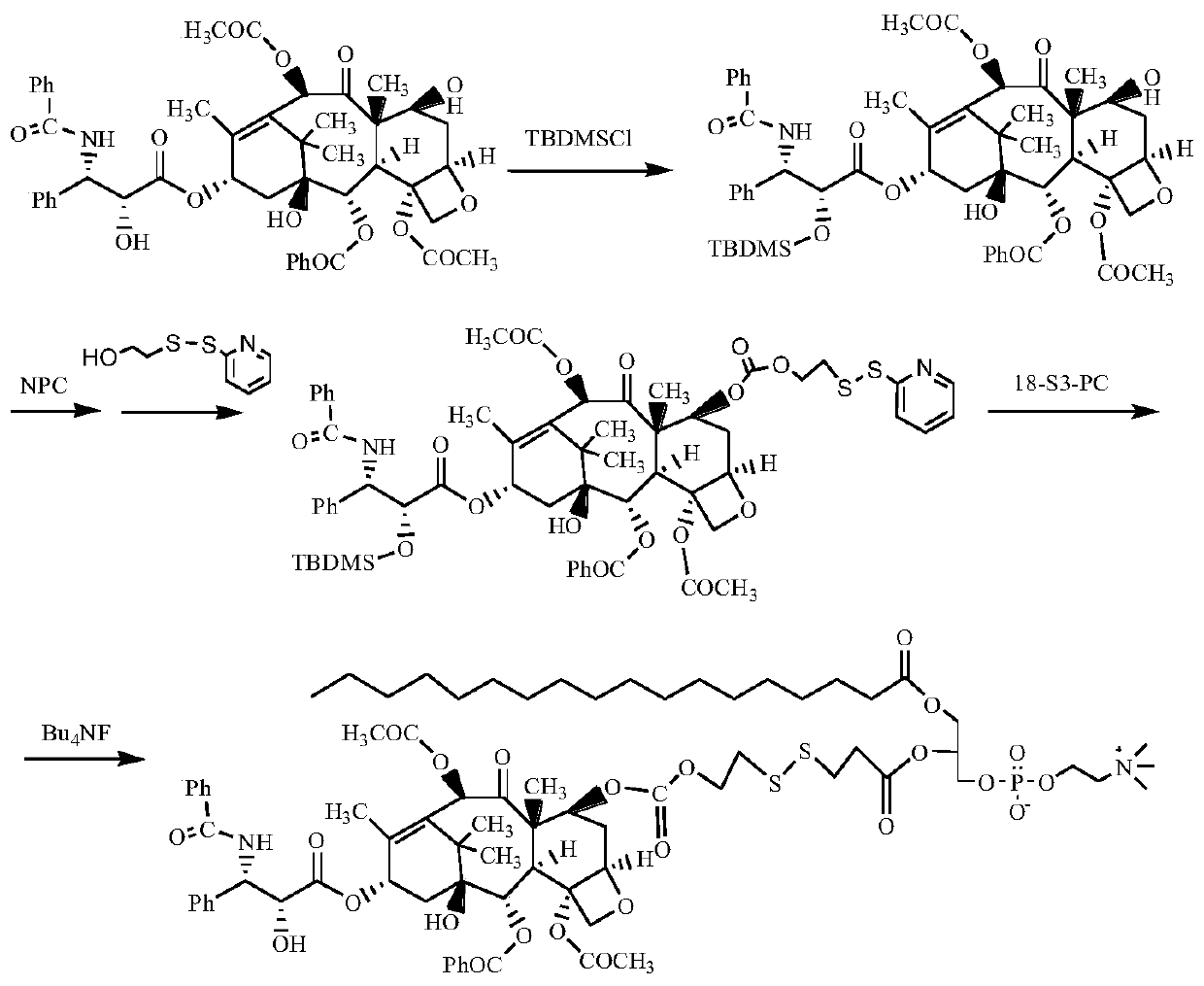
[0091] Synthesis of stearic acid-sn-2-paclitaxel-7-carboxylate ethyl dithiopropionate glycerophosphocholine (route see figure 1 )
[0092] (1) 2'-OH protection of paclitaxel (2'-TBDMS-PTX)
[0093] Add 0.6g of paclitaxel, 0.5g of imidazole, and 30mL of DMF into a 100mL round bottom flask, stir to dissolve. 1.2 g of tert-butyldimethylchlorosilane was slowly added dropwise thereto, and after 20 minutes of completion of the dropwise addition, the reaction was continued at room temperature for 12 hours. After the reaction was completed, 100 mL of dichloromethane was added for extraction and fully washed with deionized water. The organic phase was dried over anhydrous magnesium sulfate and concentrated by rotary evaporation. The product was purified by column chromatography to obtain 0.62 g of white solid 2'-tert-butyldimethylsiloxane-paclitaxel (2'-TBDMS-PTX), with a yield of 95%.
[0094] 1 H NMR (500MHz, CDCl 3 ):δ0.46(s,6H,Si(CH 3 ) 2 ),0.83(s,9H,tert-butyl),1.17(s,3H,H...
Embodiment 3
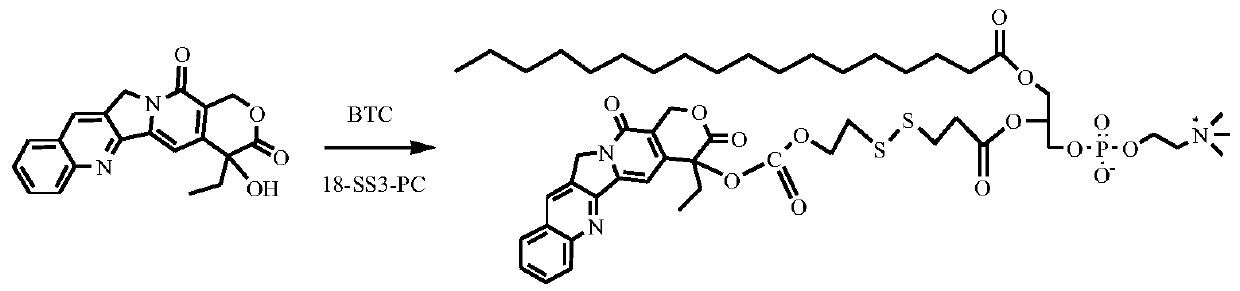
[0100] Synthesis of stearic acid-sn-2-camptothecin formate ethyl dithiopropionate glycerophosphocholine (route see figure 2 )
[0101] 0.53g of camptothecin and 0.20g of triphosgene (BTC) were mixed uniformly in 100mL of anhydrous dichloromethane. Under a nitrogen atmosphere, 0.30 g of DMAP in dichloromethane solution was slowly added dropwise, and the reaction was stirred at room temperature for 2 h. Add 0.50 g of stearic acid-sn-2-hydroxyethyldithiopropanoic acid glycerophosphocholine to the above system at one time, and react overnight. Afterwards, the crude product, MgSO 4 The organic phase was dried and concentrated. The product was purified by column chromatography to obtain 0.35 g of the product stearic acid-sn-2-camptothecin formate ethyl dithiopropionyl glycerophosphocholine as a yellow solid.
[0102] 1 H NMR (500MHz, CD3OD:CDCl 3 1:1):δ8.06-7.42(5H,m),6.74(1H,s),4.75-4.64(3H,m),4.46-4.22(6H,d),3.97-3.77(4H,d), 3.43 (2H, t), 3.30 (9H, s), 2.95-2.25 (6H, m), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com