A kind of amphiphilic molecule containing o-nitrobenzyl ester photodegradation group and its synthesis method
A technology of o-nitrobenzyl ester and amphiphilic molecules, which is applied in the field of synthesis of new light-controlled surface active molecules, can solve the problems of low yield and achieve the effects of high yield, mild reaction conditions and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Synthesis of diethylenetriaminepentaacetic acid dianhydride (DTPAA)
[0040] Add 2g of DTPA, 2.5mL of anhydrous acetic anhydride, and 3.5mL of anhydrous pyridine into a 50mL single-necked flask. Under the protection of nitrogen, heat and reflux at 65°C. After 24 hours of reaction, a dark brown suspension is obtained. Filter the filter cake with anhydrous Wash with acetic anhydride and anhydrous ether alternately until colorless, and dry in vacuum at 80°C for 12 hours to obtain 1.51 g of white solid with a melting range of 178°C to 181°C and a yield of 83.2%.
[0041] (2) Demethylene reaction:
[0042]Dissolve 1g of 6-nitropiperonal in 10mL of organic solvent, add dropwise into 2.5g of aluminum chloride organic solvent, and react for 2h under ice-cooling. After the reaction was completed, the reaction solution was poured into 25 mL of hydrobromic acid, and stirred at room temperature for 48 h. The reaction mixture was diluted with a large amount of water, extracted...
Embodiment 2
[0050] (1) Synthesis of diethylenetriaminepentaacetic acid dianhydride (DTPAA)
[0051] With embodiment 1.
[0052] (2) Demethylene reaction
[0053] With embodiment 1.
[0054] (3) etherification reaction:
[0055] Add the alkali solution and 3.97g of dodecane bromide into the organic solution of 1g of 4,5-dihydroxy-2-nitrobenzaldehyde, and react at 60°C for 15h under the protection of nitrogen. The reaction mixture was diluted with a large amount of water, extracted three times with ether, dried over anhydrous magnesium sulfate, and the filtrate obtained by suction filtration was spin-dried, recrystallized in a solvent and filtered by suction to obtain a yellow solid 4,5-didodecyloxy-2-nitro Benzaldehyde 2.12g, the yield is 79.4%.
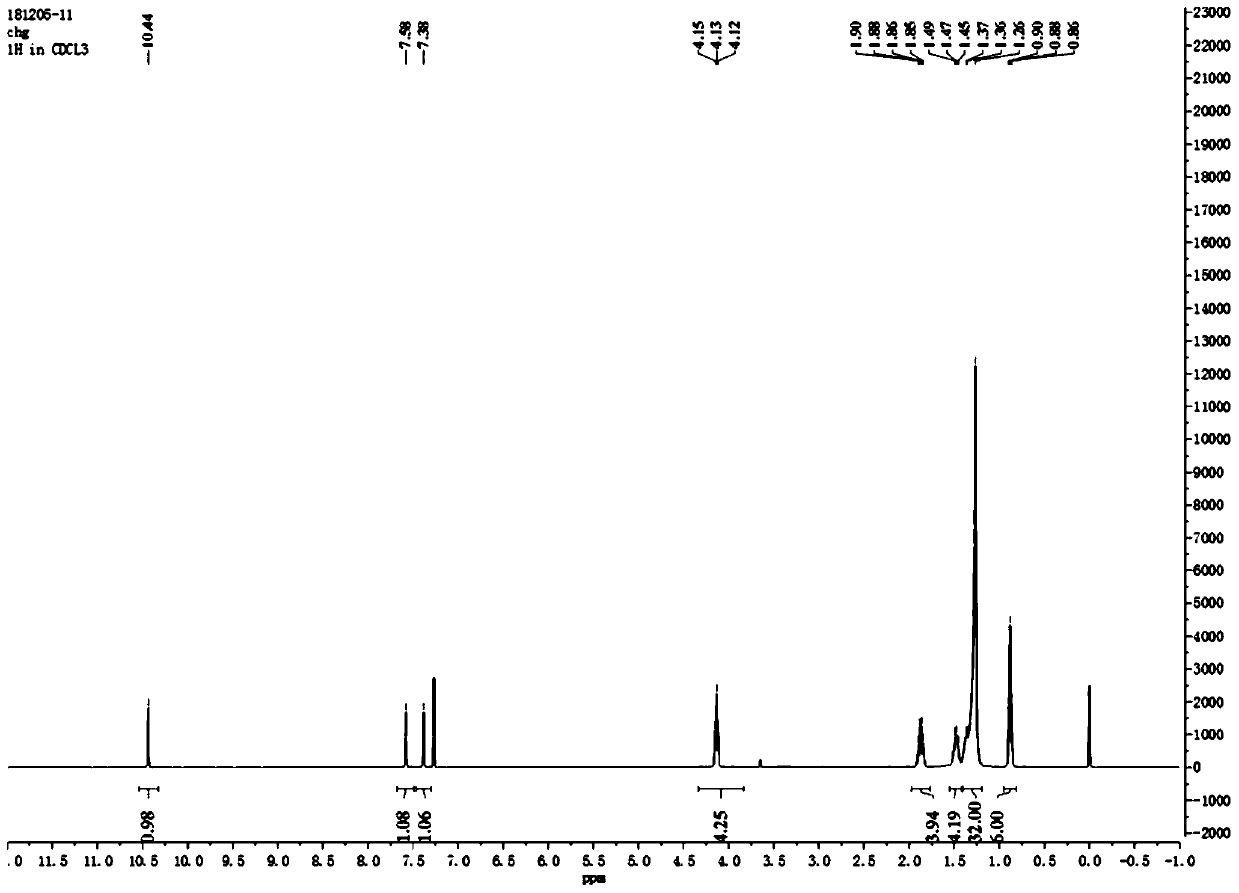
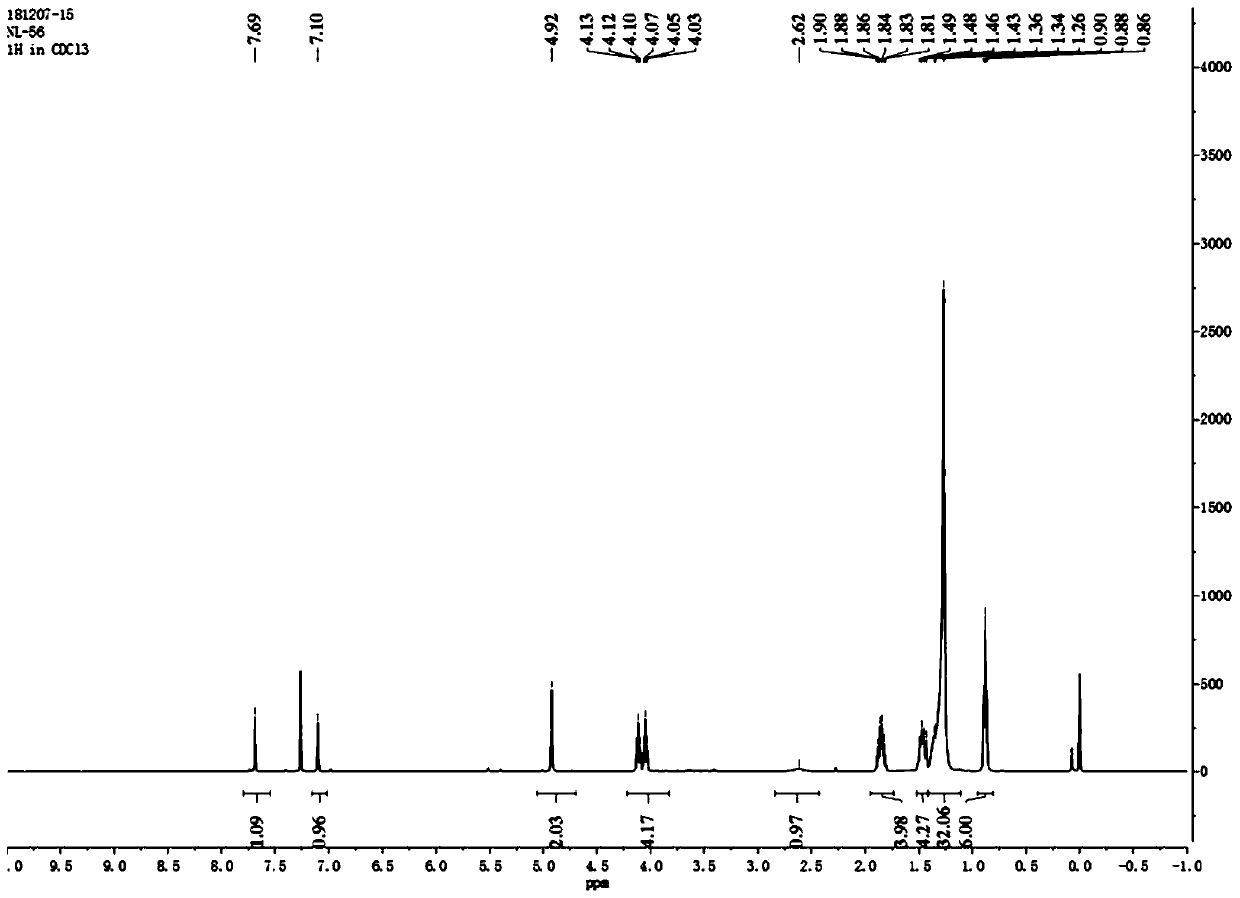
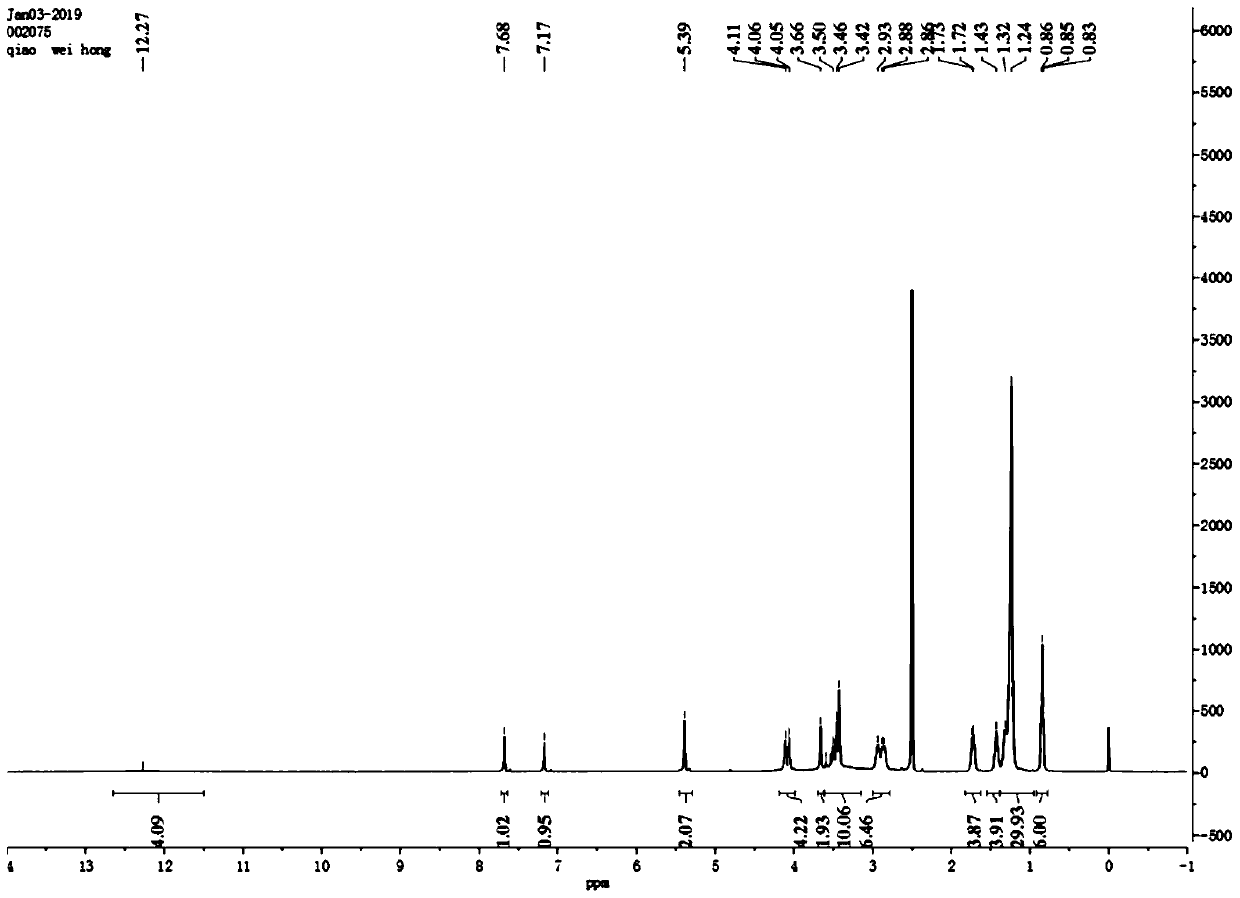
[0056] Such as figure 1 As shown, the 4,5-didodecyloxy-2-nitrobenzaldehyde 1 H NMR diagram, 1 H-NMR (500MHz, CDCl 3 ): δin ppm0.86-0.90(t,6H,-CH 2 -CH 3 ); 1.26-1.49(m,36H,-(CH 2 ) 9 -CH 3 ); 1.85-1.90 (m,4H,-O-CH 2 -CH 2 -); 4.10-...
Embodiment 3
[0066] (1) Synthesis of diethylenetriaminepentaacetic acid dianhydride (DTPAA)
[0067] With embodiment 1.
[0068] (2) Demethylene reaction
[0069] With embodiment 1.
[0070] (3) etherification reaction:
[0071] Add the alkali solution and 5.45 g of bromooctadecane to the organic solution of 1 g of 4,5-dihydroxy-2-nitrobenzaldehyde, and react at 60° C. for 15 h under the protection of nitrogen. The reaction mixture was diluted with a large amount of water, extracted three times with ether, dried over anhydrous magnesium sulfate, and the obtained filtrate was spin-dried, recrystallized in a solvent and filtered to obtain a yellow solid 4,5-dioctadecyloxy-2-nitro Benzaldehyde 2.82g, the yield is 74.9%.
[0072] (4) Reduction reaction:
[0073] Add 0.55g of sodium borohydride to 2g of 4,5-dioctadecyloxy-2-nitrobenzaldehyde in a mixed solution of methanol and tetrahydrofuran, react at -7~0°C for 30min, and react at room temperature for 3h. After the reaction was complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com