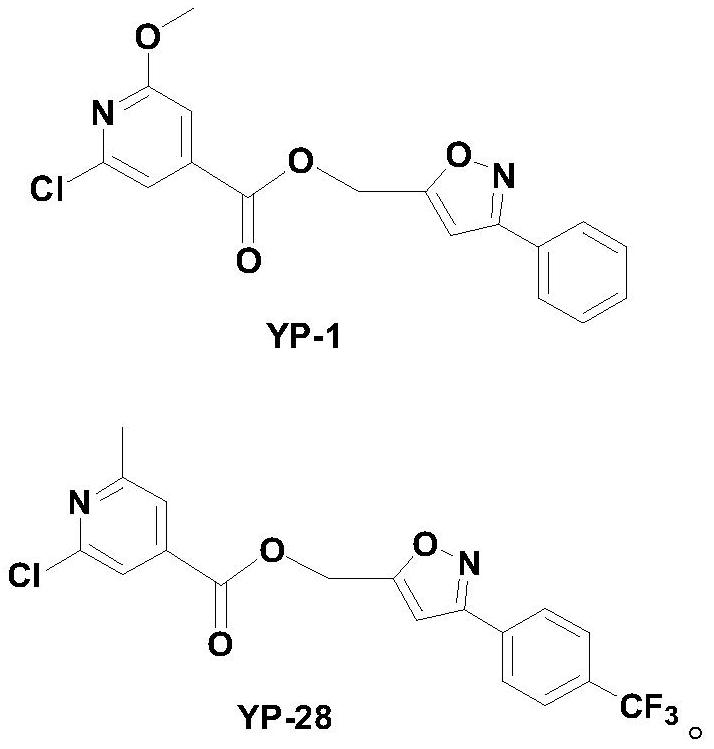
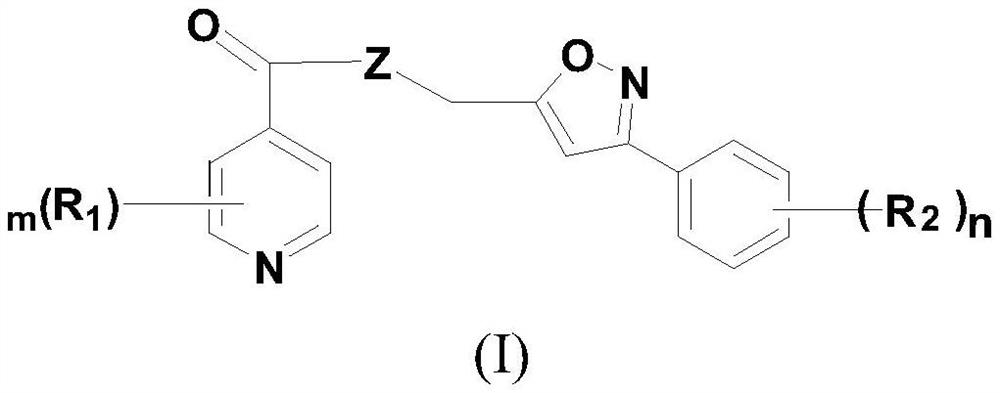
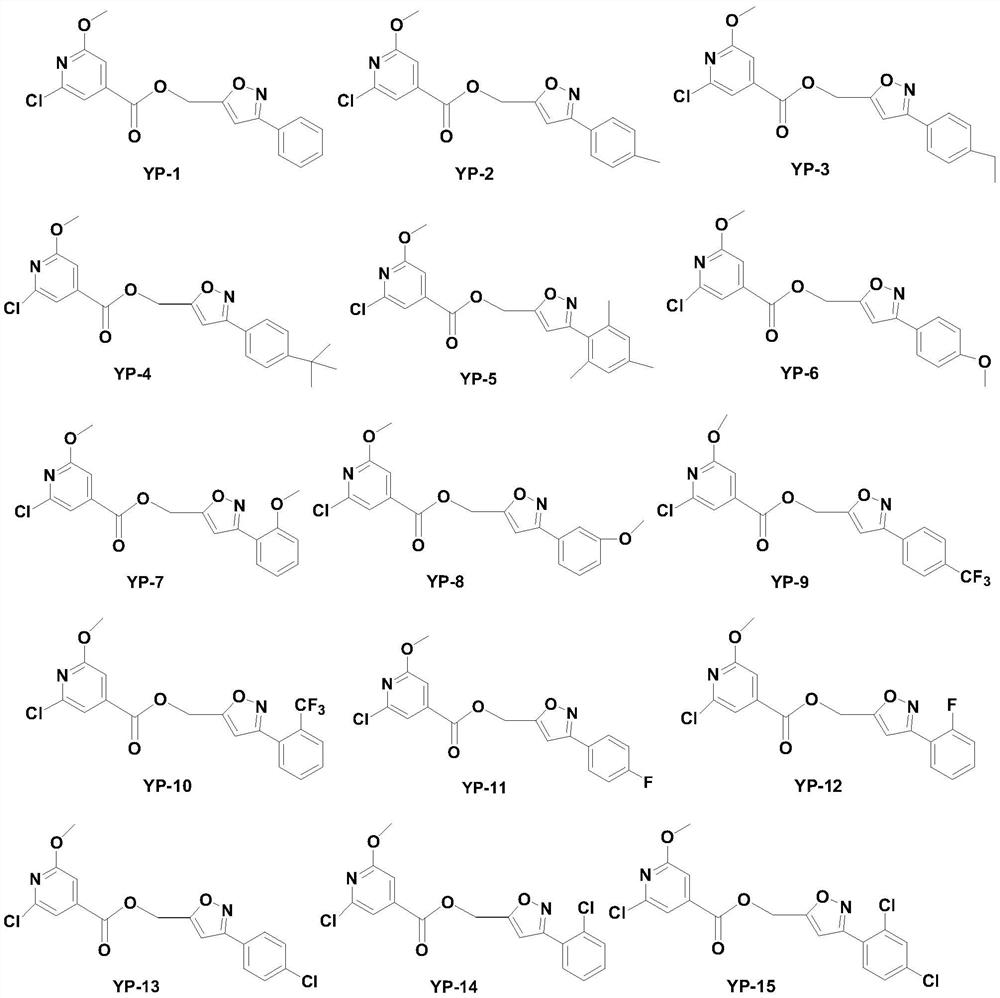
Isonicotinic acid derivative and its preparation method and use
A derivative, isonicotinic acid technology, applied in the field of isonicotinic acid amide derivatives and their preparation, isonicotinic acid derivatives, isonicotinic acid ester derivatives, can solve the problem of affecting physiological functions, cancer cell proliferation and recurrence, Injury to normal cells and other problems, to achieve a broad-spectrum anti-cancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Synthesis of embodiment 1 intermediate 3-substituted phenyl-5-hydroxymethyl-isoxazole (II-1) or intermediate 3-substituted phenyl-5-aminomethyl-isoxazole (II-2) :
[0080] Using substituted benzaldehyde as raw material, it is prepared by synthesis of oxime, 1,3-dipolar cycloaddition reaction, methylsulfonyl esterification reaction, azidation and reduction reaction (R 2 and n as defined above), see the following process for details:
[0081]
[0082] The specific synthetic process of intermediate 3-substituted phenyl-5-hydroxymethyl-isoxazole (II-1) or intermediate 3-substituted phenyl-5-aminomethyl-isoxazole (II-2) is detailed See the three applications of the applicant with publication numbers CN103360382A, CN103664991A and CN103601762A, the full texts of which are incorporated herein by reference.
Embodiment 2
[0083] Synthesis of isonicotinic acid lipid derivatives shown in embodiment 2 formula (I)
[0084] Wherein the reaction of isonicotinic acid and 3-phenyl-5-hydroxymethyl-isoxazole is exemplified:
[0085] Synthesis of [(3-phenyl-isoxazol-5-yl)-methyl]-pyridine-3-carboxylate (YP-77)
[0086]
[0087] Add 0.123g (1mmol) of nicotinic acid and 0.206g (1mmol) of DCC into a 50mL round bottom flask, add 10mL of dry THF, stir and react in an ice bath for 30min, and dissolve 0.175g (1mmol) of 5-hydroxymethyl-3- Phenyl-isoxazole and 0.122 g (1 mmol) of DMAP in 10 mL THF were slowly added dropwise to the reaction system, stirred in an ice bath for 30 min, and then naturally rose to room temperature for reaction. After the TLC detection reaction was completed, the reaction solution was concentrated in vacuo, and the residue was directly separated by column (V (石油醚) :V (乙酸乙酯) =5:1~2:1) to obtain the target compound [(3-phenyl-isoxazol-5-yl)-methyl]-pyridine-3-carboxylate (YP-77).
...
Embodiment 3
[0103] Embodiment 3 biological activity test
[0104] MTT method was used to test the activity of the specific compound against colorectal cancer cell line HCT-116, human lung cancer cell line A549 and breast cancer cell line MCF-7. The specific test process is as follows:
[0105] (1) Spread the lung cancer cell line A549 in a 96-well plate, add 100 μL of culture medium, and wait for the cells to grow to 90%, add 1 μL of drugs into the wells, and detect 8 different concentrations of each drug (respectively, the initial concentration of the drug Concentration, 50μM, 5μM, 500nM, 50nM, 5nM, 500pM, 50pM), for each drug concentration, do 3 replicate wells in parallel, after culturing for 18h, add 20μL of the prepared 5mg / mL MTT solution to each well for 4 hours Afterwards, the medium was aspirated, and 150 μL of DMSO was added to each well, and the optical density (OD) value of the compound to be tested was measured at a wavelength of 595 nm. The negative control is DMSO. The i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com