Method for preparing bis((3, 4-epoxycyclohexyl) methyl) adipate by using microchannel reactor
A micro-channel reactor, epoxy cyclohexyl technology, applied in organic chemistry and other directions, can solve the problems of continuous production difficulty, low product yield, high reaction temperature, and achieve high practical value, high product purity, and reaction yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
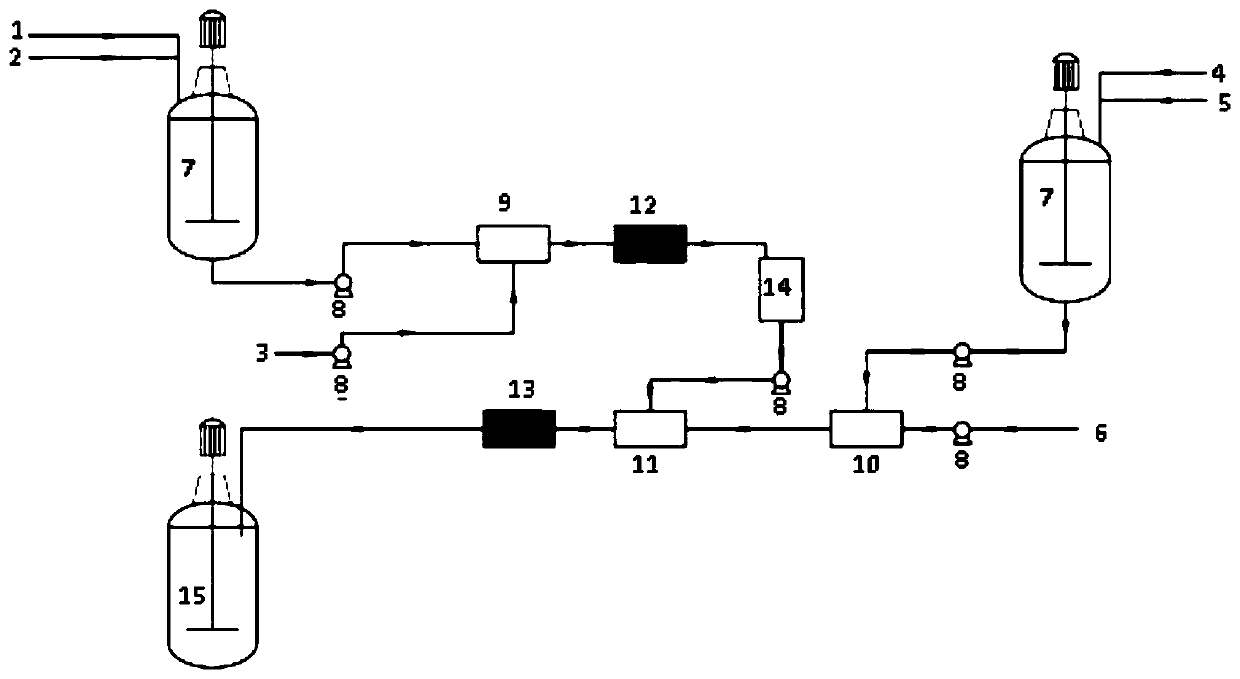
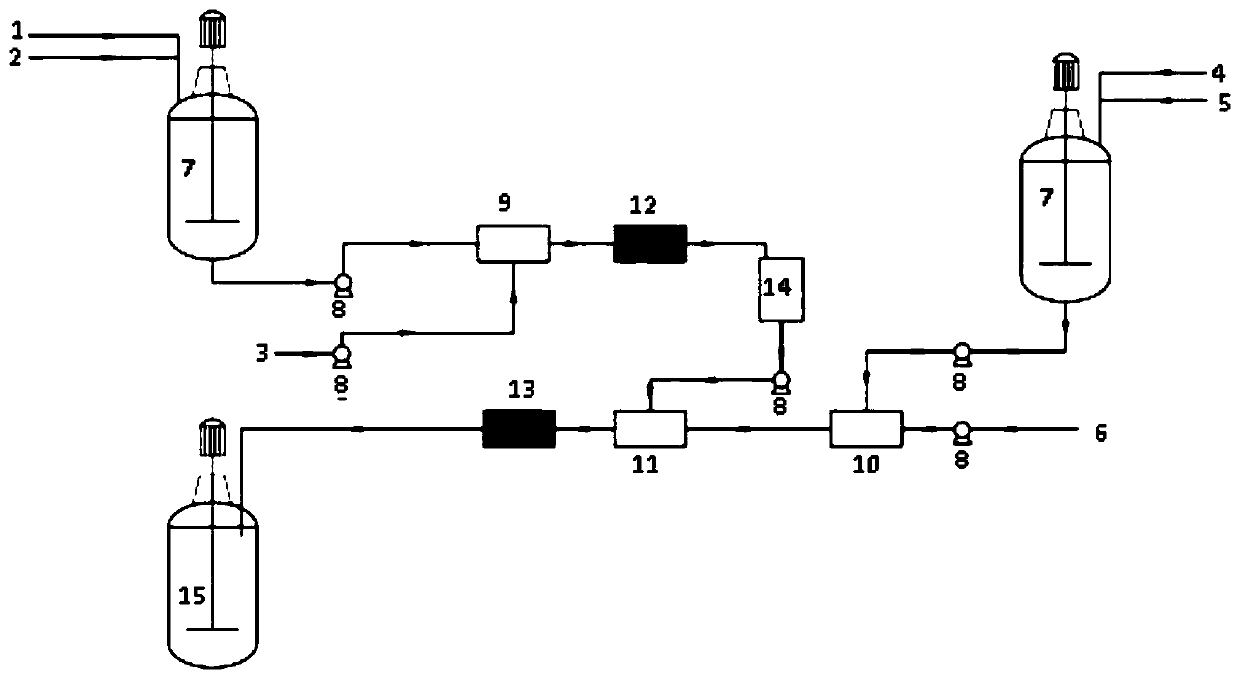
Image
Examples
Embodiment 1
[0044] Esterification reaction: 114.5g of 3-cyclohexene-1-methanol (98%), 102.2g of triethylamine (99%) and 400g of tetrahydrofuran were sequentially added into a three-necked flask and stirred evenly to obtain solution 1. Solution 1 and 102.7g hexadienoyl chloride (98%) were pumped into the first mixer at the ratio of flow rate 8.7mL / min: 1mL / min and mixed, then entered into the first microchannel reactor, at a temperature of 40°C Next, react for 30 minutes. After the reaction finishes, filter the salts that reaction generates, and remove solvent and obtain intermediate two ((3-cyclohexenyl) methyl) adipate 324.3g, purity 98.1%, molar yield is 95.2% (according to Calculate the amount of adipoyl chloride).
[0045]Epoxidation reaction: 324.3 g of bis((3-cyclohexenyl)methyl)adipate obtained above was dissolved in 650 g of chloroform to obtain solution 2. 37.7g of anhydrous sodium acetate was dissolved in 321g of 50% hydrogen peroxide (w / W) aqueous solution to obtain solution ...
Embodiment 2
[0049] Esterification reaction: 114.5g of 3-cyclohexene-1-methanol (98%), 102.2g of triethylamine (99%) and 650g of chloroform were sequentially added into a three-necked flask and stirred evenly to obtain solution 1. Solution 1 and 102.7g hexadienoyl chloride (98%) were pumped into the first mixer at the ratio of flow rate 8.7mL / min: 1mL / min and mixed, then entered into the first microchannel reactor, at a temperature of 40°C Next, react for 30 minutes. After the reaction, filter the salts generated by the reaction to obtain the intermediate bis((3-cyclohexenyl)methyl)adipate solution (solution two); this step solution can be directly used without vacuum distillation. In the epoxidation reaction, the operation steps are simplified.
[0050] Epoxidation reaction: 37.6g of anhydrous sodium acetate was dissolved in 320g of 50% hydrogen peroxide (w / W) aqueous solution to obtain solution three; solution three and 310g of acetic anhydride were mixed at a flow rate of 5.1mL / min: 5m...
Embodiment 3
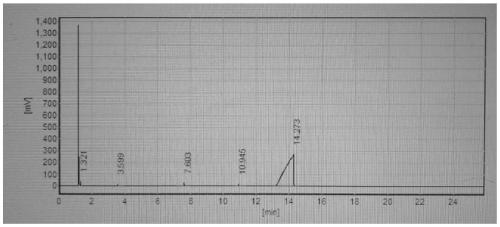
[0053] Implementation conditions and steps are the same as in Example 2, except that the triethylamine in the esterification reaction is changed into 32% aqueous sodium hydroxide solution, and the purity of bis((3,4-epoxycyclohexyl)methyl)adipate is 97.8%. %, the molar yield is 93.4%, and the epoxy equivalent value is 205.1g / eq.
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy equivalent | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com