A biotin-targeted photosensitizer and quercetin nano-delivery system
A technology of biotin and nano-assembly, which is applied in drug combination, antineoplastic drugs, pharmaceutical formulations, etc., can solve the problems of low bioavailability and poor water solubility, and achieve targeting effect, water solubility improvement, and technical effect improvement Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The second aspect of the present disclosure provides a preparation method of the compound described in the first aspect, the preparation method comprising the following steps:
[0035] (1) IR780 reacts with 3-amino-1-propanol to obtain IR780-OH;
[0036] (2) reacting the IR780-OH with biotin to obtain the compound described in the first aspect.
[0037] In some embodiments, the step (1) and / or (2) is a catalytic reaction, and the catalytic reaction is enzymatic catalysis or chemical catalysis; preferably, it is a chemical catalysis reaction.
[0038] In some embodiments, the IR780 in step (1) reacts with 3-amino-1-propanol under the condition of triethylamine.
[0039]Preferably, the concrete operation of described step (1) is as follows:
[0040] Dissolve IR780 in anhydrous N,N-dimethylformamide (DMF), add triethylamine, and react with 3-amino-1-propanol under inert gas protection and oil bath conditions to prepare the intermediate product IR780-OH.
[0041] Furthe...
Embodiment 1B780
[0064] Embodiment 1 B780 molecular synthesis
[0065] Weigh a certain amount of IR780 with an analytical balance, dissolve them in anhydrous N, N-dimethylformamide (DMF) and place them in a y round-bottomed flask, add triethylamine and 3-amino-1 -propanol, under nitrogen protection, and react in an oil bath at 85° C. for 4 h, wherein the molar weight is IR780: 3-amino-1-propanol: triethylamine = 1:5:0.6. After the reaction was completed, the anhydrous DMF was removed by rotary evaporation under reduced pressure, and the crude product was obtained by vacuum drying overnight. The crude product was dissolved in methanol, mixed with silica gel for a little column chromatography, purified by silica gel column chromatography, and gradient eluted with dichloromethane and methanol (100:1), to obtain pure IR780-OH as a blue solid. Weigh a certain amount of biotin and dissolve it in 5mL of anhydrous DMF, slowly drop DMAP and EDCI into the above solution under stirring conditions, activ...
Embodiment 2
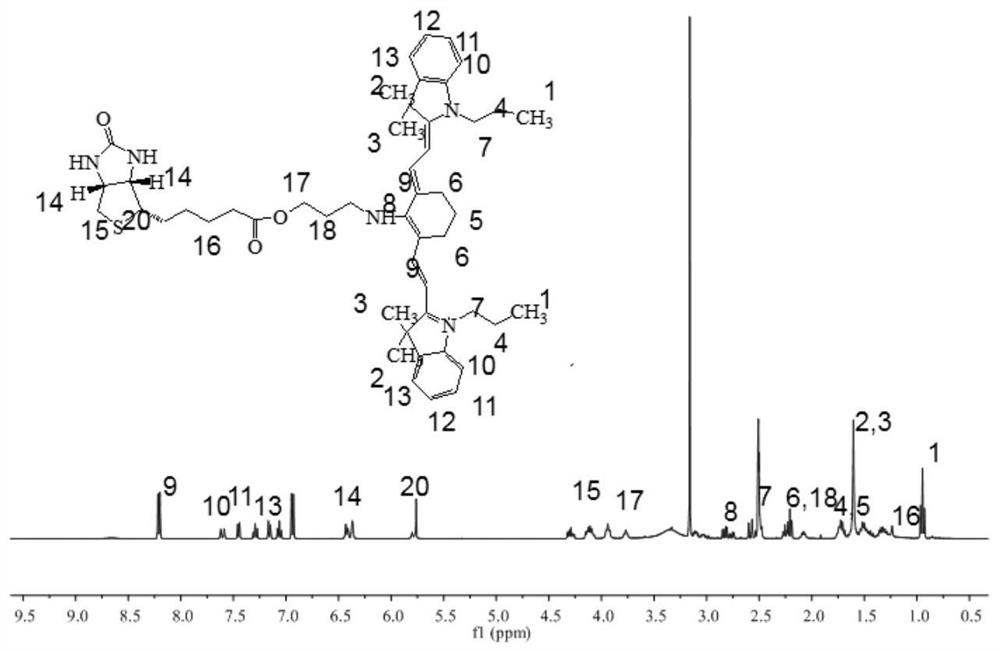
[0066] Embodiment 2 Proton nuclear magnetic resonance spectrum ( 1 H-NMR) to identify the chemical structure of B780
[0067] About 5 mg of B780 was weighed, dissolved in deuterated dimethyl sulfoxide (DMSO-d6) and placed in an NMR tube, and its H NMR spectrum was measured by 400 MHz H NMR spectrum, and the chemical shift value (ppm) of the compound was recorded. The result is as figure 1 As shown, the NMR results can confirm that the molar ratio of IR780-OH and biotin in the newly synthesized molecule is close to 1:1, which can confirm the successful synthesis of B780.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com