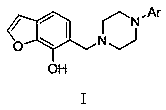
6-piperazinemethyl-7-hydroxybenzofuran compound and medical application thereof
A compound and hydroxybenzene technology, applied in the field of compounds and their medicinal uses, can solve problems such as difficulty in controlling intestinal symptoms, and achieve the effect of a simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
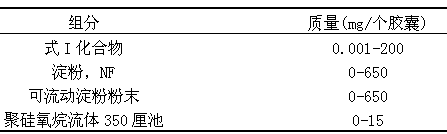
Examples
Embodiment 1
[0063] Example 1: Preparation of 6-[(4-phenylpiperazin-1-yl)methyl]-7-hydroxybenzofuran (T01):
[0064] Add 4.0 g (0.030 mol) of 7-hydroxybenzofuran, 4.9 g (0.030 mol) of 1-phenylpiperazine, 1.0 g (0.033 mol) of paraformaldehyde, and 1 mL of glacial acetic acid into the reaction flask, use an appropriate amount of ethanol as a solvent, and heat Reflux for 4~8h, TLC monitors the reaction process, after the reaction is completed, cool down, and remove ethanol by rotary evaporation to obtain a yellow oil, which is separated and purified by column chromatography with ethyl acetate:petroleum ether (1:5) as eluent, The solvent was removed by rotary evaporation, and 2.6 g of a light yellow solid was precipitated by freezing, with a yield of 28.2%. ESI-MS m / z: 309.2; 1 H-NMR (CDCl 3 ) δ(ppm): 2.62-2.68 (4H, m), 3.16-3.22 (4H, m), 3.78 (2H, s), 6.70(1H, m), 6.80(1H, d, J =8.1 Hz), 6.98-7.08(2H, m), 7.16-7.22 (4H, m),7.68(1H, d, J =8.1 Hz). Example 2: Preparation of 6-{[4-(4-methyl...
Embodiment 2
[0065] According to the preparation method of Example 1, a light yellow solid was obtained with a yield of 26.1%. ESI-MS m / z: 351.4; 1 H-NMR (CDCl 3 ) δ(ppm): 2.27 (3H, s), 2.65-2.70 (4H, m), 3.20-3.32 (4H, m), 3.80 (2H, s), 6.70(1H, d, J =8.1 Hz), 6.96 (2H, d, J = 8.2 Hz), 7.00-7.06(2H, m), 7.16(2H, d, J =8.2 Hz), 7.72(1H, d, J =8.1 Hz).
Embodiment 3
[0066] Example 3: Preparation of 6-{[4-(2-methylphenyl)piperazin-1-yl]methyl}-7-hydroxybenzofuran (T03):
[0067] According to the preparation method of Example 1, a light yellow solid was obtained with a yield of 24.9%. ESI-MS m / z: 351.4; 1 H-NMR (CDCl 3 ) δ(ppm): 2.28 (3H, s), 2.58-2.66 (4H, m), 3.25-3.34 (4H, m), 3.86 (2H, s), 6.56-6.65 (1H, m), 6.77(1H , d, J =8.1 Hz), 6.94-7.03(3H, m), 7.08-7.13(2H,m), 7.82(1H, d, J =8.1 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com