Gamma-hydroxy ketone derivatives and a synthesis method thereof
A synthetic method and technology of hydroxy ketones, applied in the field of γ-hydroxy ketone derivatives, achieving the effects of mild reaction conditions, easy availability of raw materials, and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
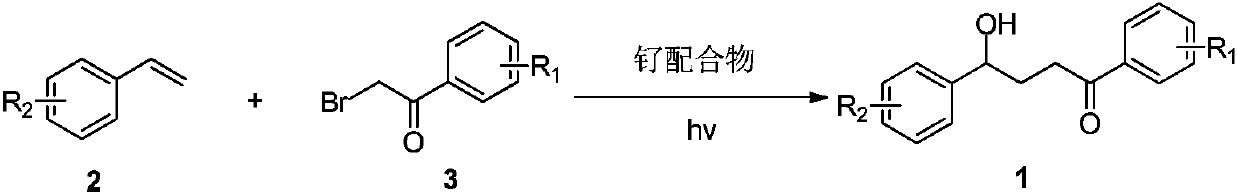
[0030] The specific process is: in the glove box, take p-methylstyrene 2a (35mg, 0.3mmol), Ru(bpy) 3 Cl 2 ·6H 2 O (4 mg, 0.006 mmol), α-bromoacetophenone 3a (120 mg, 0.6 mmol), NaHCO 3 (25mg, 0.3mmol) was added to a 25mL tube with a branch, and acetonitrile (3mL) and water (18mg, 0.3mmol) were added under a nitrogen atmosphere and reacted at room temperature for 24h under the irradiation of a 26W white CFL lamp. After the reaction was complete, the solvent was removed by rotary evaporation under reduced pressure, and then column chromatography (petroleum ether (60-90 ℃) / ethyl acetate: 20:1, v / v) obtained a light yellow liquid product 1a (53 mg, yield 70 %). The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 2
[0032]
[0033] The specific process is: weigh 1a (76mg, 0.3mmol) in the glove box, add sodium azide (20mg, 0.3mmol) into a 25mL reaction tube with a branch, and 2 Under protection, 3 mL of 1,4-dioxane and boron trifluoride diethyl ether (55 mg, 0.39 mmol) were added, and reacted at 90° C. for 4 h. The solvent was spun off, column chromatography: PE(60-90°C) / EA=10 / 1, v / v. The pale yellow liquid 4a (17 mg, yield 20%) was obtained. The target product was confirmed by NMR and high-resolution mass spectrometry.
[0034] Typical Compound Characterization Data
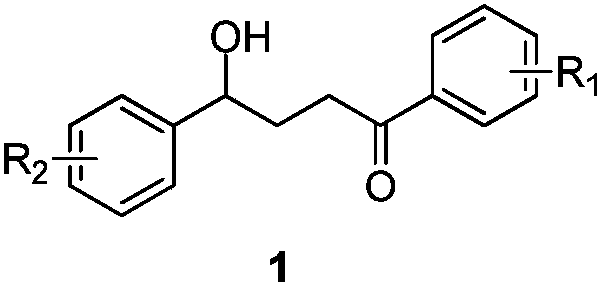
[0035] 4-Hydroxy-1,4-diphenyl-1-butanone derivative (1a), pale yellow liquid. 1 H NMR (400MHz, CDCl 3 )δ8.02–7.85(m,2H),7.58–7.48(m,1H),7.42(dd,J=10.6,4.7Hz, 2H),7.25(d,J=8.0Hz,2H),7.14(d ,J=7.9Hz,2H),4.77(t,J=6.3Hz,1H),3.08(t,J=7.0Hz,2H),2.56(s,1H),2.33(s,3H),2.24– 2.09(m,2H). 13 C NMR (100MHz, CDCl 3 )δ200.7(s), 141.5(s), 137.3(s), 136.9(s), 133.2(s), 129.3(s), 128.7(s), 128.2(s), 125.8(s), 73.5( s), 34.9(s), 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com