No-wash aggregation-inducible cell membrane targeting staining reagent based on purine skeleton and its preparation method and application
An aggregation-induced, cell membrane technology, applied in the preparation of test samples, styryl dyes, chemical instruments and methods, etc., can solve the problems of low accuracy of imaging results, complicated operation process, inability to connect sensing, etc., to avoid Interference from background light, economical availability of raw materials, and low overall cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
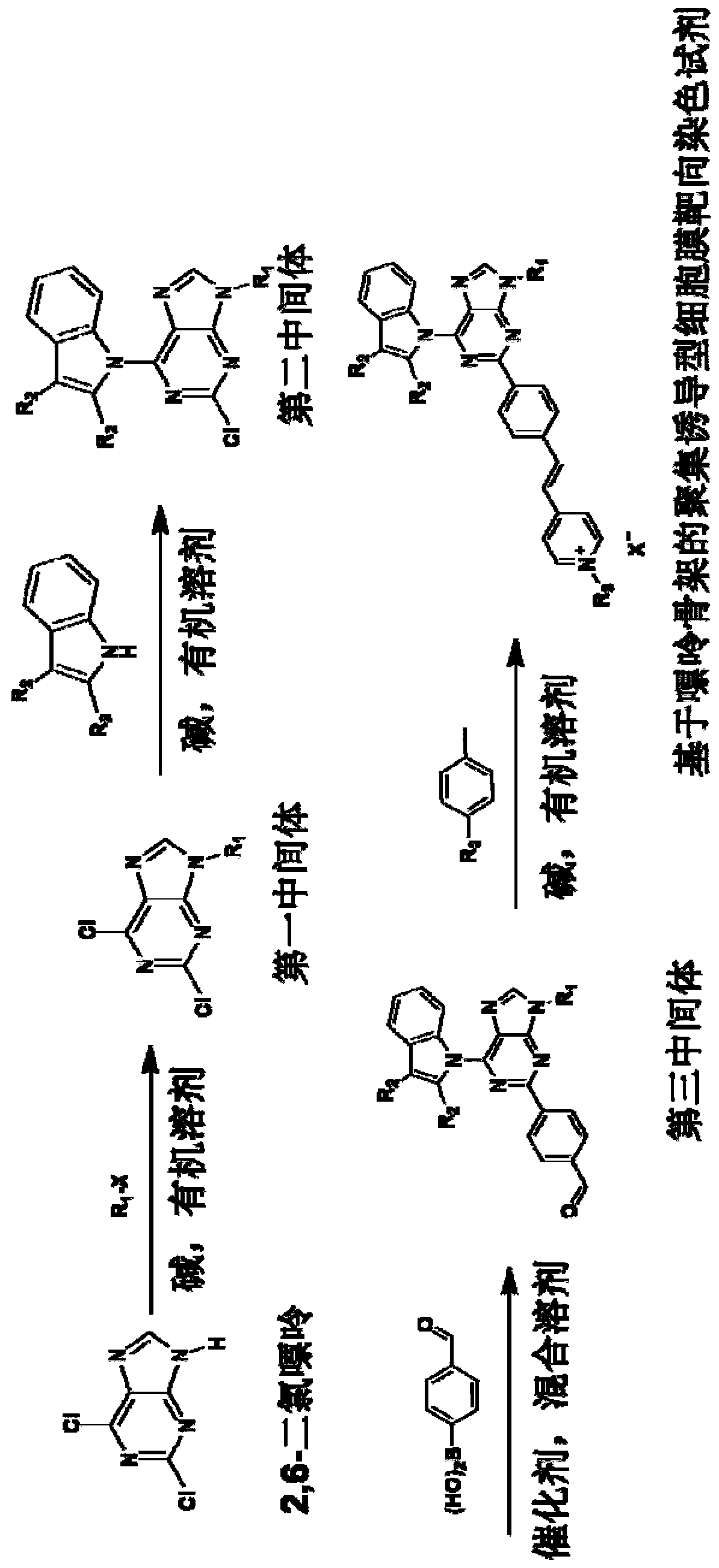
[0067] The preparation method of the purine skeleton-based aggregation-inducible cell membrane targeting staining reagent of this embodiment comprises the following steps:
[0068] (1) Synthesis of the first intermediate: 2,6-dichloro-9-n-propyl-9-hydrogen-purine
[0069] The synthetic route is as follows:
[0070]
[0071] 2,6-Dichloropurine (1.0 mmol), 1-bromopropane (1.5 mol) and potassium carbonate (3.0 mmol) were mixed and stirred in DMSO (5 mL) for 6 hours, then 100 mL of water was added. The organic layer was separated, and the aqueous layer was extracted with ethyl acetate (30 mL×3). The organic extracts were washed with brine and treated with Na 2 SO 4 dry. After the solvent was removed and distilled under reduced pressure, it was purified by 200-300 mesh silica gel column chromatography. Elution with petroleum ether / ethyl acetate (3:2) gave the first intermediate as a white solid with a yield of 57%. The eluent is ethyl acetate / petroleum ether=2:3 (V / V). Fi...
Embodiment 2
[0090] This example is basically the same as Example 1, except that the third intermediate R is changed 3 The substituent, its synthetic route is as follows:
[0091]
[0092]After adding the third intermediate (381 mg, 1 mmol) and 1,4-lutidine-1-iodide (235 mg, 1 mmol) in ethanol (10 mL), piperidine (0.05 mL) was dropped into the stirring liquid. Then the mixture was stirred at room temperature for about 12 hours. After the completion of the reaction was monitored by thin-layer chromatography, the solvent was removed by rotary evaporation, and then dissolved with saturated potassium hexafluorophosphate acetone solution (10 mL). After stirring at room temperature for 2 hours, the acetone was distilled off under reduced pressure, and then the crude product was obtained by filtration. The crude product was purified by neutral alumina column chromatography. Elution with methanol / dichloromethane=20 / 1 (V:V) gave a yellow solid with a yield of 37%.
[0093] 1 H NMR (400MHz, D...
Embodiment 3
[0096] This example is basically the same as Example 1, except that the picoline salt in step (4) is changed to 4-methyl-1-(3-(trimethylammonium) propyl)pyridinium-1-ammonium bromide , its synthetic route is as follows:
[0097]
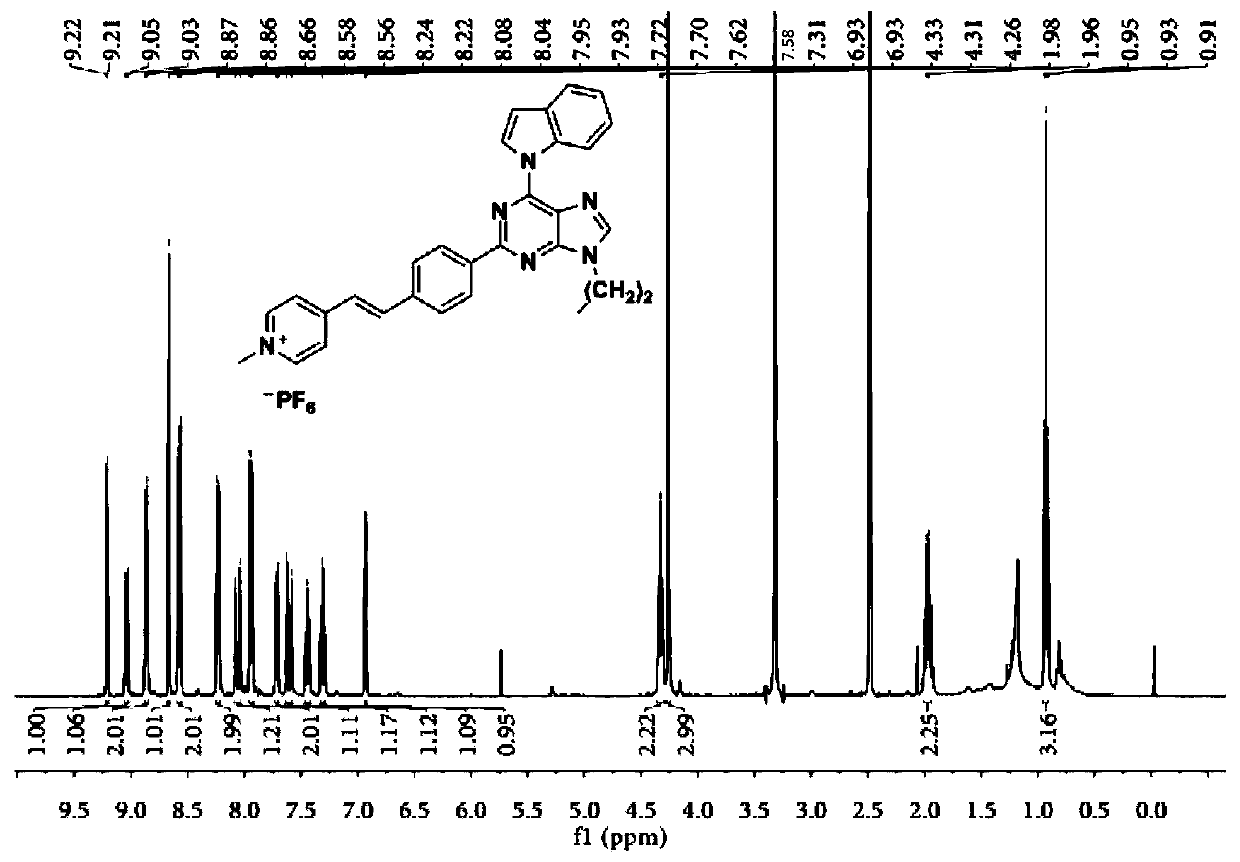
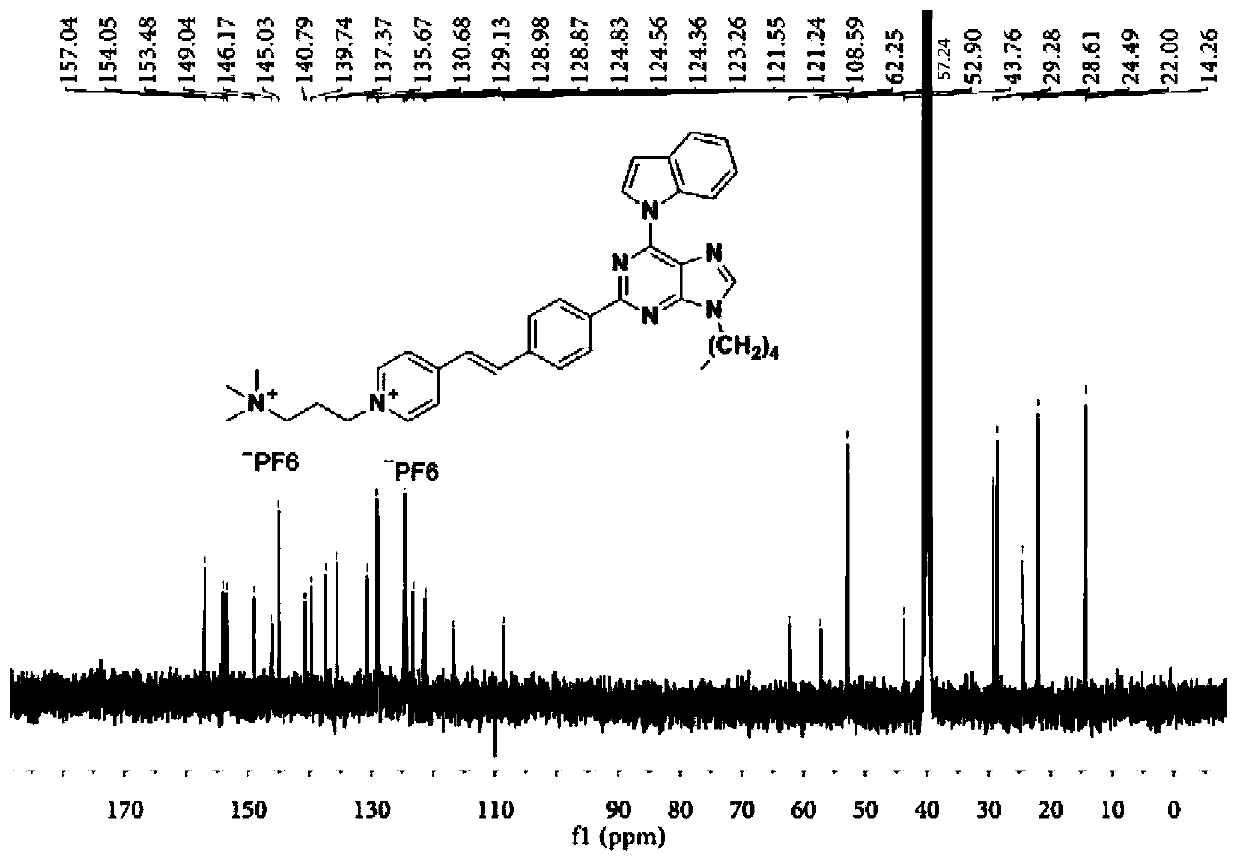
[0098] A dark brown solid was obtained in 33% yield. 1 H NMR (400MHz, DMSO-d 6 )δ9.23-9.20(d,1H),9.09-9.03(m,3H),8.71-8.69(s,1H),8.62-8.57(d,2H),8.37-8.33(d,2H),8.20- 8.14(d,1H),8.02-7.98(d,2H),7.74-7.67(m,2H),7.47-7.42(t,1H),7.34-7.28(t,1H),6.95-6.93(d,1H ),4.65-4.60(t,2H),4.40-4.34(t,2H),3.11-3.7(s,9H),1.99-1.92(m,2H),0.89-0.83(t,3H). 13 C NMR (101MHz, DMSO-d 6 )δ157.08,154.10,153.50,149.06,146.25,145.04,140.83,139.78,137.37,135.68,130.69,129.15,129.00,128.93,124.83,124.58,124.36,123.26,121.57,121.29,116.71,109.99,108.61,62.25,57.24 ,52.91,45.50,24.47,23.06,11.51.HRMS(ESI):m / z:Calcd for C 35 h 39 f 6 N 7 P + :702.2903; [M-PF 6 ] + Found: 702.2902.
[0099] The hydrogen spectrum, carbon spectrum and high-resolution mass spectrum of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com