Fluorescent probe for detecting GSH with high specificity and application thereof
A fluorescent probe, high specificity technology, applied in fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., can solve the problems of indistinguishability, and achieve the effect of simple method, good biocompatibility, and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
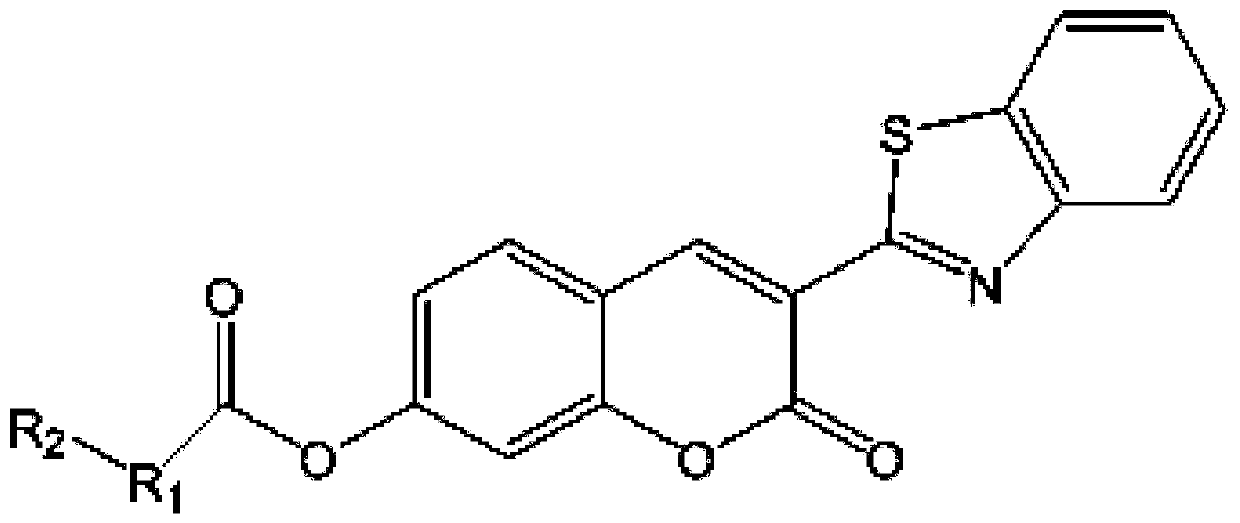
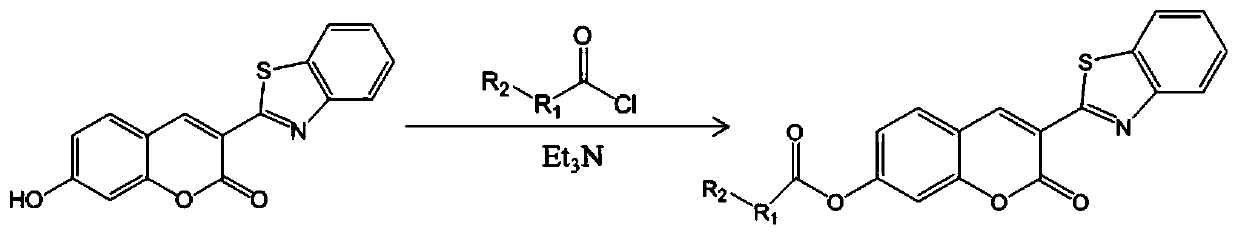
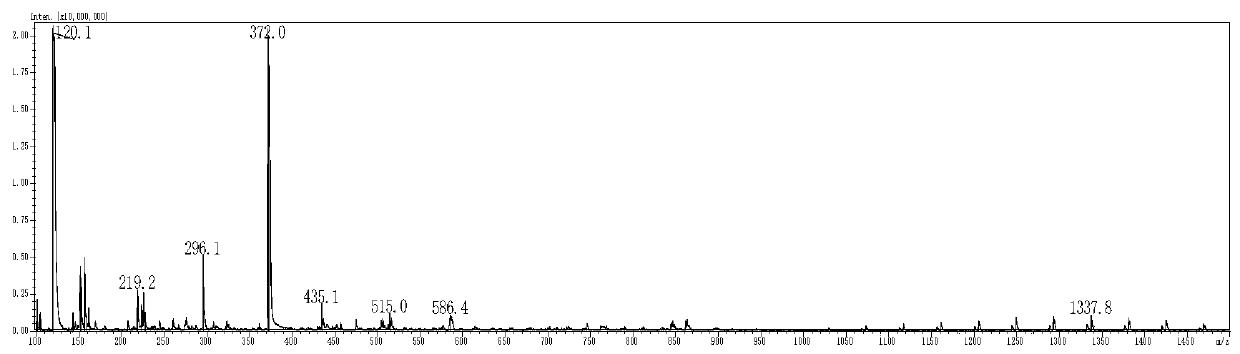
[0049] A fluorescent probe for highly specific detection of GSH, such as figure 1 As shown, the fluorescent probe is 3-benzothiazolyl-coumarin-7-phenol ester derivatives, wherein, R 1 is methyl, R 2 for the chlorine atom. Such as figure 2 As shown, the fluorescent probe uses 3-benzothiazolyl-7-hydroxycoumarin compounds as raw materials to obtain 3-benzothiazolyl-coumarin-7-phenol ester derivatives through esterification . 3-benzothiazolyl-7-hydroxycoumarin is used as a raw material, and 2-chloroacetic acid is esterified to obtain 2-chloroacetic acid 3-benzothiazolyl-coumarin-7-phenol ester. Such as image 3 , Figure 6 As shown, we have successfully prepared 2-chloroacetic acid 3-benzothiazolyl-coumarin-7-phenol ester.
[0050] Compounds with a coumarin core structure all have good fluorescence emission spectrum characteristics (450-650nm). The present invention connects coumarin and benzothiazole as a novel fluorescent probe, through the generation and quenching of fl...
Embodiment 2
[0052] A fluorescent probe for highly specific detection of GSH, such as figure 1 As shown, the fluorescent probe is 3-benzothiazolyl-coumarin-7-phenol ester derivatives, wherein, R 1 is methyl, R 2 for the chlorine atom. Such as figure 2 As shown, the fluorescent probe uses 3-benzothiazolyl-7-hydroxycoumarin compounds as raw materials to obtain 3-benzothiazolyl-coumarin-7-phenol ester derivatives through esterification . 3-benzothiazolyl-7-hydroxycoumarin is used as a raw material, and 3-chloropropionic acid is esterified to obtain 3-benzothiazolyl-coumarin-7-phenol ester of 3-chloropropionic acid. Such as Figure 4 , Figure 7 As shown, we have successfully prepared 3-chloropropionic acid 3-benzothiazolyl-coumarin-7-phenol ester.
[0053] Compounds with a coumarin core structure all have good fluorescence emission spectrum characteristics (450-650nm). The present invention connects coumarin and benzothiazole as a novel fluorescent probe, through the generation and qu...
Embodiment 3
[0055] A fluorescent probe for highly specific detection of GSH, such as figure 1 As shown, the fluorescent probe is 3-benzothiazolyl-coumarin-7-phenol ester derivatives, wherein, R 1 is methyl, R 2 for the chlorine atom. Such as figure 2 As shown, the fluorescent probe uses 3-benzothiazolyl-7-hydroxycoumarin compounds as raw materials to obtain 3-benzothiazolyl-coumarin-7-phenol ester derivatives through esterification . 3-benzothiazolyl-7-hydroxycoumarin is used as a raw material, and is reacted with 3-bromopropionic acid to obtain 3-benzothiazolyl-coumarin-7-phenol ester of 3-bromopropionic acid. Such as Figure 5 , Figure 8 As shown, we have successfully prepared 3-benzothiazolyl-coumarin-7-phenol 3-bromopropionate.
[0056] Compounds with a coumarin core structure all have good fluorescence emission spectrum characteristics (450-650nm). The present invention connects coumarin and benzothiazole as a novel fluorescent probe, through the generation and quenching of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com