Flexible liposome cosmetic containing active small molecular substances and preparation method thereof
A technology of small molecular substances and flexible liposomes, which is applied in the direction of cosmetic preparations, cosmetics, dressing preparations, etc., to achieve the effect of improving the effect and resisting ultraviolet rays
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1 Preparation of flexible liposomes containing active small molecular substances of the present invention
[0066] The preparation technology of the flexible liposome containing water-soluble and fat-soluble small molecular substances is described as follows:
[0067] 1) Flexible liposomes containing water-soluble small molecular substances:
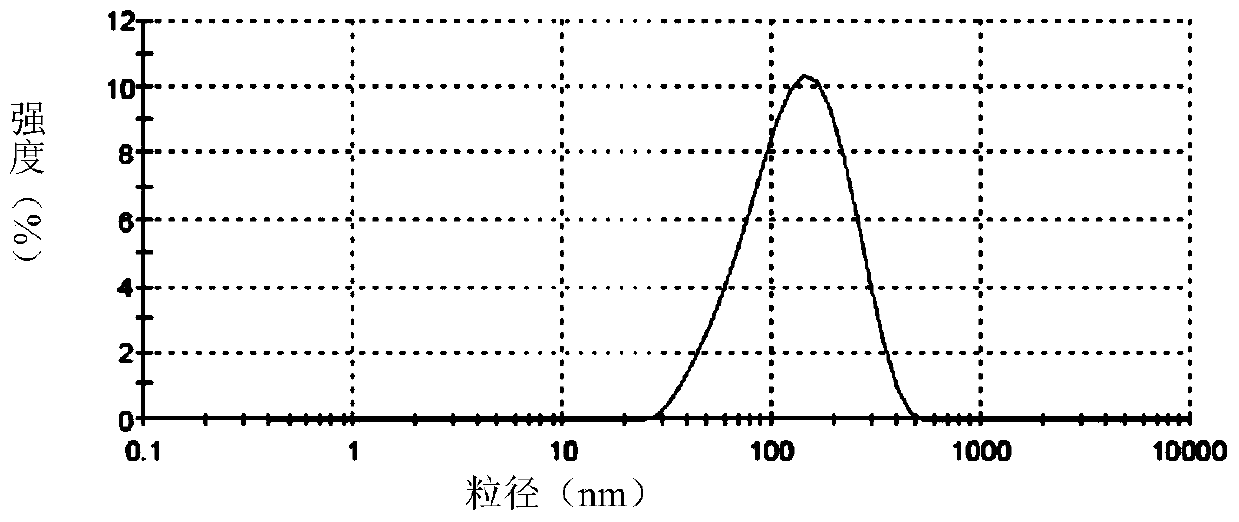
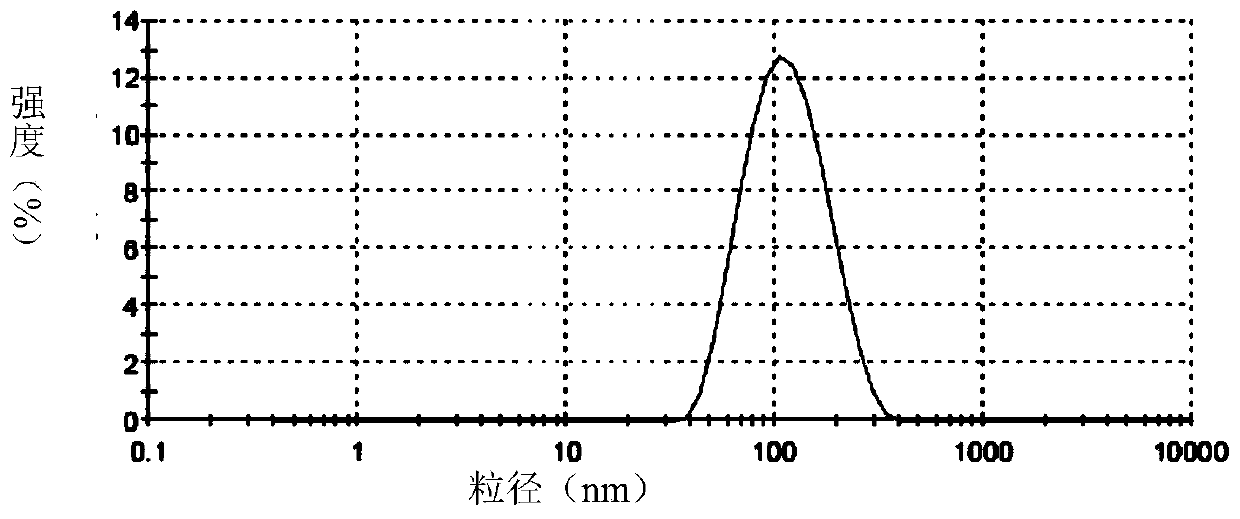
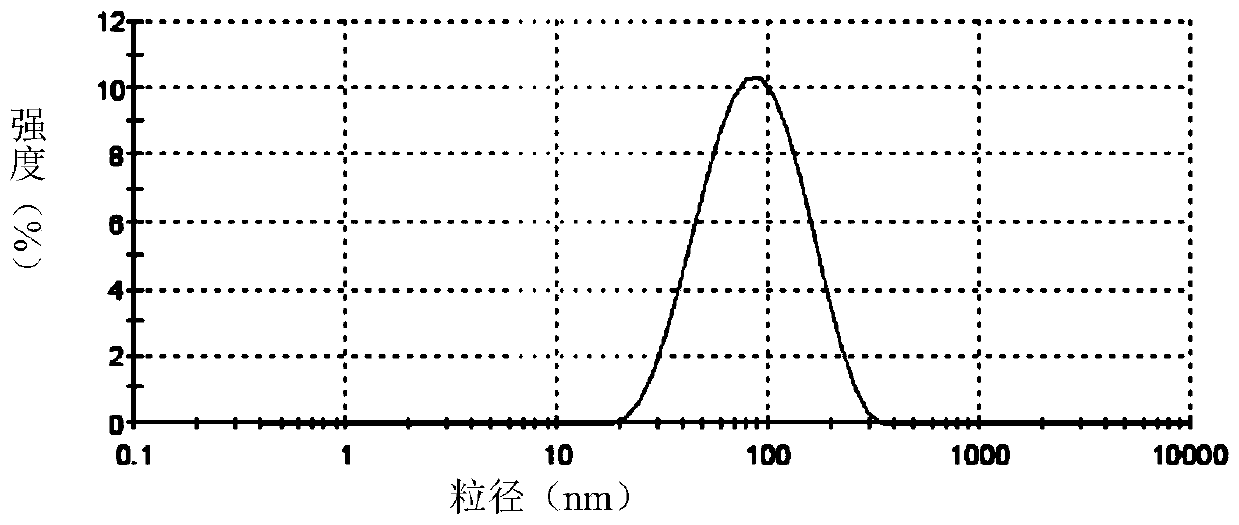
[0068] Accurately weigh soybean lecithin, sodium deoxycholate or polysorbate 80 or its derivatives, vitamin E according to the formula, dissolve with a mixture of chloroform and ethanol or only ethanol, dissolve and mix in a 250ml pear-shaped bottle, and store at room temperature Rotary evaporated for 2 hours, and the resulting film was placed in a vacuum oven overnight. The next day, distilled water and appropriate amount of hydrophobically modified polypeptide DP7-C or PAL-DP7 were added, and a 400w probe was sonicated for 30 minutes to obtain a blank transfersome solution. Slowly add an appropriate amount of water-...
Embodiment 2
[0138] Example 2 Verification of the transdermal effect of the flexible liposome containing active small molecules of the present invention
[0139] The in vitro transdermal experiment adopts a modified single-chamber Franz diffusion cell, and mouse skin is used as the in vitro transdermal experimental skin to compare the transdermal absorption results of different flexible liposomes containing active small molecules, and samples are taken at different time points. The concentration of the drug in the transdermal receiving solution was determined by HPLC method, and the cumulative transdermal amount was calculated.
[0140] Test example 2-1 Verification of the transdermal effect of flexible liposomes containing nicotinamide
[0141] 1. Materials
[0142] Samples: lyophilized powder of flexible liposomes containing nicotinamide prepared in Example 1 (1-1, 1-2, 1-3, 1-4) and nicotinamide-containing liposomes not modified by DP7-C or PAL-DP7 The flexible liposome of amide is us...
Embodiment 3
[0177] Example 3 Stability study of the flexible liposome freeze-dried powder containing active small molecules of the present invention
[0178] The flexible liposome freeze-dried powder containing active small molecules prepared in Example 1 was subjected to stability research (including three aspects such as accelerated test and long-term test). The samples were placed in a drug stability test chamber at 37°C for accelerated testing, and placed for 1, 2, 3, and 6 months respectively, and the properties of the freeze-dried powder were measured, including color, content, and solubility. The results are shown in Table 7. Then put the samples in a drug stability test box at 25°C for long-term tests, and place them for 3, 6, 9, 12, 15, 18, 21, and 24 months respectively to detect the color, HPLC content, and solubility of the freeze-dried powder. The results are shown in Table 8.
[0179] The stability accelerated test (37 ℃) of table 7 lyophilized powder
[0180]
[0181] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com