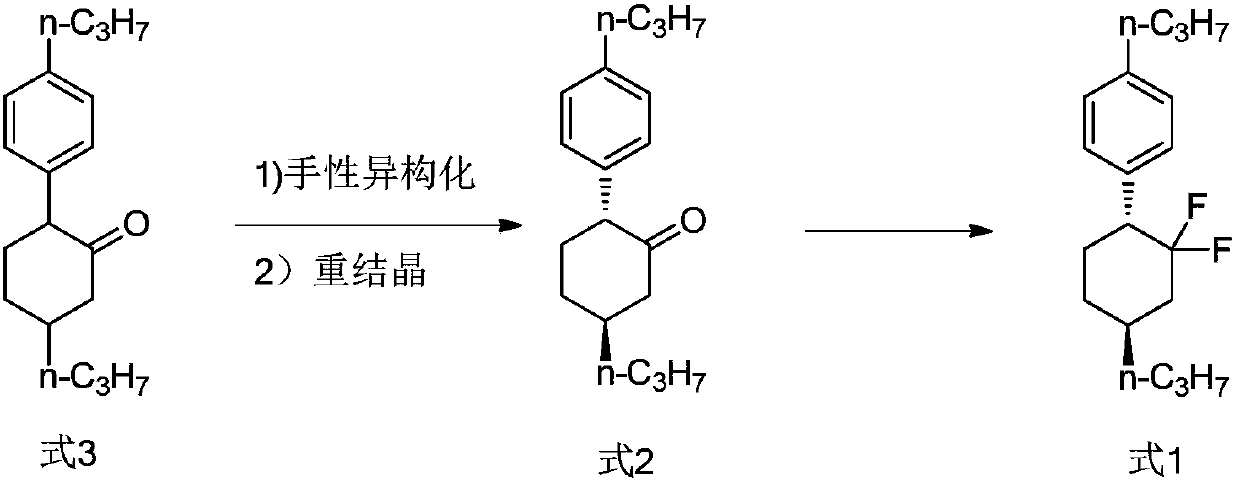
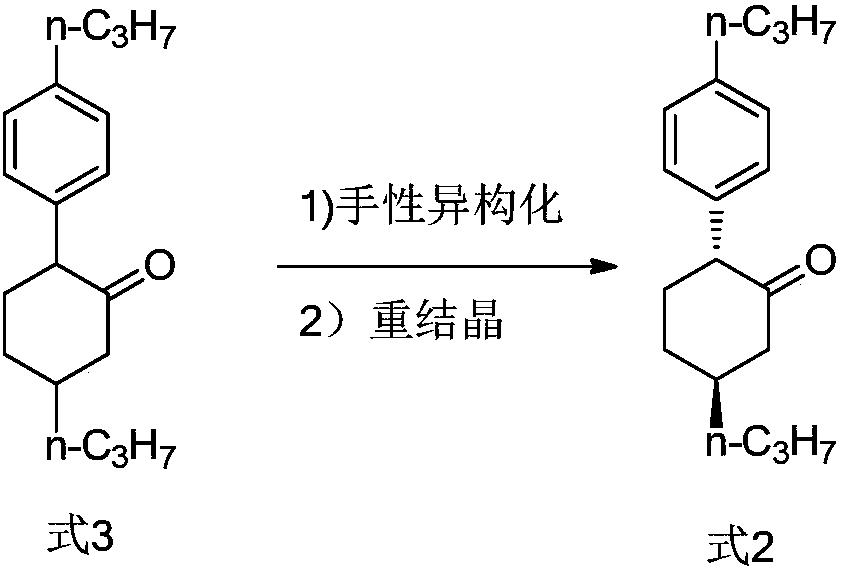
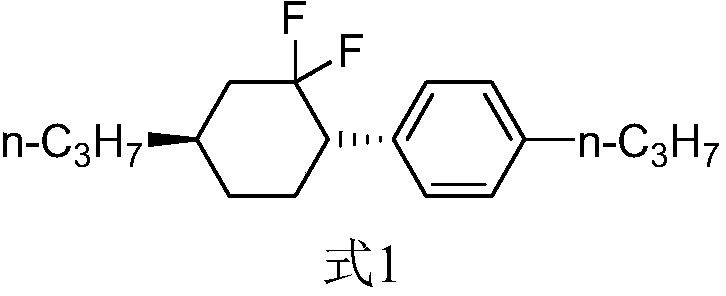
Method of preparing high-purity trans-1-(2,2-difluoro-4-propylcyclohexyl)-4-propylbenzene
A technology of propylcyclohexanone and propylbenzene, which is applied in the field of preparation of liquid crystal materials, can solve the problems of low proportion of chiral isomerization mixture, difficulty in obtaining high purity, low proportion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The preparation method of the present invention involves the chiral isomerization of 2-(4-propylphenyl)-5-propylcyclohexanone (compound of formula 3) under Lewis acid catalysis. Those skilled in the art know that there are literature-related patents in the prior art that mention the chiral isomerization of cyclohexane (without side chain ketone groups) with a base at high temperature. However, the 2-(4-propylphenyl)-5-propylcyclohexanone (compound of formula 3) involved in the present invention is different from the literature reports, and its cyclohexane structure contains a carbonyl group. The present inventors found that the ideal chiral isomerization reaction could not be achieved under high temperature, base catalysis or alcohol solvent, base catalysis. On the contrary, at high temperature without solvent or under alkali catalysis, the chiral isomerization product develops to a ratio of 50:50, and the reaction at high temperature produces more degradation impuritie...
Embodiment 1
[0067] According to the reaction process shown above, the inventors have investigated the use of potassium carbonate, sodium ethylate, sodium methylate, sodium hydroxide, potassium hydroxide, potassium tert-butoxide, anhydrous lithium chloride, anhydrous aluminum trichloride, three Comparative experiment of chiral isomerization of ketone body formula 3 with fluoromethanesulfonic acid, trifluoroacetic acid, anhydrous zinc chloride, and 15% sulfuric acid.
[0068] Table 1: Chiral isomerization reactions with different catalysts.
[0069]
[0070] Through comparative experiments, referring to patents and other literature reports, the effect of chiral isomerization in alkali is not high, and the chiral isomerization in the opposite direction is obtained under the catalysis of some alkalis. The cis-body in the product becomes larger; unexpectedly, it is found that this compound has better chiral isomerization in Lewis acid, and anhydrous aluminum trichloride has the best chiral ...
Embodiment 2
[0072] In this embodiment, the inventor further investigated the catalyst is aluminum trichloride, in the solvent dichloromethane, chloroform, 1,2-dichloroethane, acetonitrile, THF or toluene chiral isomerization reaction comparison .
[0073] Table 2: Chiral isomerization reaction of catalyst aluminum trichloride in different solvents.
[0074]
[0075] Through comparative experiments, the ratio of chiral isomerization in dichloromethane is the highest, and the yield is the highest.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com